<ol id="33bym"></ol> 

Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Chemical Description |
2-chlorobenzaldehyde is an organic compound with a chlorinated benzene ring and an aldehyde functional group. |
|
Preparation |
2-Chlorobenzaldehyde is produced mainly by chlorination of 2-chlorotoluene to form 2-chlorobenzal chloride, which is then subjected to acid hydrolysis. Metal salts, such as iron(III) chloride, are used as catalysts. The hydrolysis can also be accomplished using formic acid without a catalyst.2-Chlorobenzaldehyde can also be produced by oxidation of 2-chlorobenzyl chloride with N-oxides of tertiary amines or with dilute nitric acid.https://pubchem.ncbi.nlm.nih.gov/compound/2-Chlorobenzaldehyde |
|
Application |
2-Chlorobenzaldehyde is used acid zinc plating brightener, also be used for organic synthesis, agricultural pesticide and pharmaceutical industries. It is used to synthesize the acaricides clofentezine and flutenzine. 2-Chlorobenzaldehyde undergoes alkynylation with phenylacetylene in the presence of catalytic ligands and dimethylzinc at 0°C to form binaphthyl-derived amino alcohols. |
|
Synthesis Reference(s) |
Tetrahedron Letters, 34, p. 8037, 1993 DOI: 10.1016/S0040-4039(00)61444-2 |
|
General Description |
2-chlorobenzaldehyde is a clear colorless to yellowish liquid. (NTP, 1992) |
|
Air & Water Reactions |
2-Chlorobenzaldehyde is moisture and light sensitive. Slightly water soluble. |
|
Reactivity Profile |
2-Chlorobenzaldehyde reacts with iron and strong oxidizers, strong bases and strong reducing agents. |
|
Health Hazard |
Symptoms of exposure to 2-Chlorobenzaldehyde may include skin, eye and upper respiratory tract irritation. This compound may cause skin, eye and respiratory tract irritation. When heated to decomposition it emits toxic fumes. |
|
Fire Hazard |
2-Chlorobenzaldehyde is combustible. |
|
Safety Profile |
Poison by intraperitoneal and intravenous routes. When heated to decomposition it emits toxic fumes of Cl-. See also ALDEHYDES and CHLORIDES. |
|
Purification Methods |
Wash it with 10% Na2CO3 solution, then fractionally distil it in the presence of a small amount of catechol as stabiliser. [Beilstein 7 H 233, 7 IV 561.] |
InChI:InChI=1/C7H5ClO/c8-7-4-2-1-3-6(7)5-9/h1-5H
A simple and convenient procedure for th...
A practical method is disclosed for the ...
A selective aerobic oxidative cleavage o...
Herein, a practical and sustainable meth...
A hydroxyl radical-mediated aerobic clea...
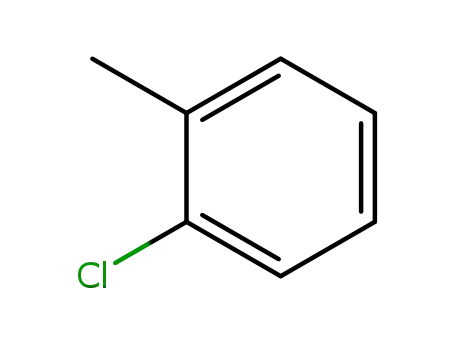
2-methylchlorobenzene

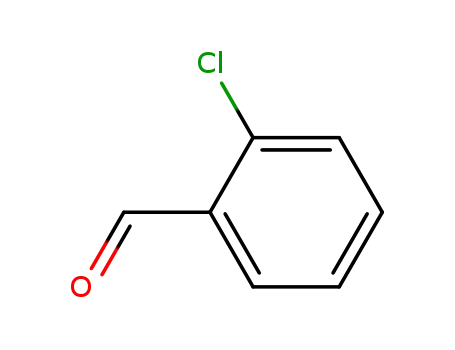
2-chloro-benzaldehyde

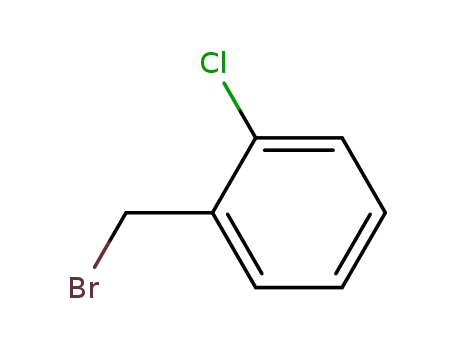
1-bromomethyl-2-chlorobenzene

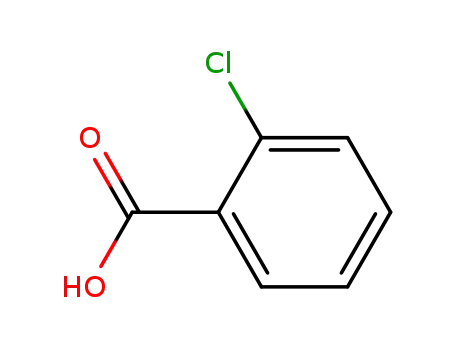
ortho-chlorobenzoic acid
| Conditions | Yield |
|---|---|
|
With
oxygen; cobalt(II) acetate; sodium bromide;
In
acetic acid;
at 95 ℃;
for 0.666667h;
Kinetics;
Mechanism;
Rate constant;
other time; other temperature; various concentrations of Co(OAc)2 and NaBr;
|
96% |
|
With
oxygen; cobalt(II) acetate; sodium bromide;
In
acetic acid;
at 95 ℃;
for 0.666667h;
|
95% |
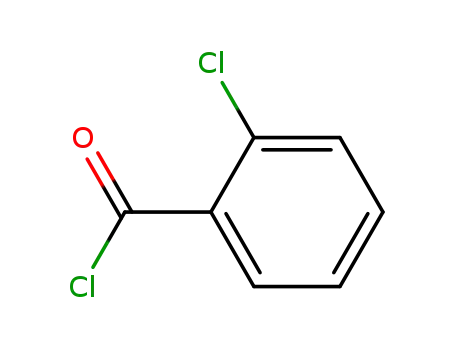
o-chlorobenzoyl chloride

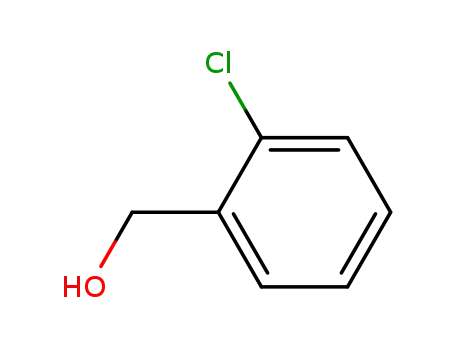
2-Chlorobenzyl alcohol


2-chloro-benzaldehyde
| Conditions | Yield |
|---|---|
|
With
pyridine; sodium tetrahydroborate;
In
tetrahydrofuran; N,N-dimethyl-formamide;
at 0 ℃;
for 0.0166667h;
Product distribution;
other borane scavengers;
|
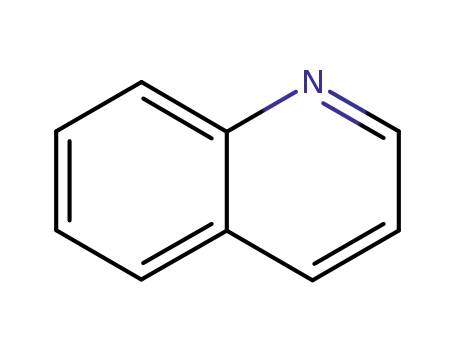
quinoline
meta-dinitrobenzene

2-Chlorobenzyl alcohol
sodium carbonate
3-(2-chlorophenyl)-2-methylacrylic acid
1-[(2-chlorophenyl)(piperidin-1-yl)methyl]naphthalen-2-ol
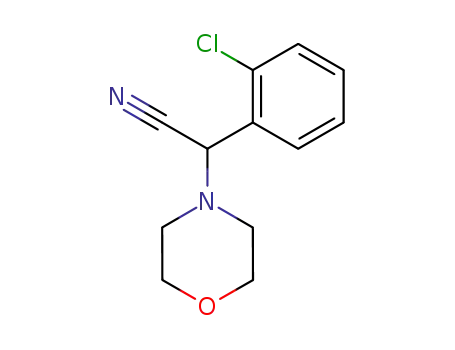
α-(2-chlorophenyl)-4-morpholineacetonitrile
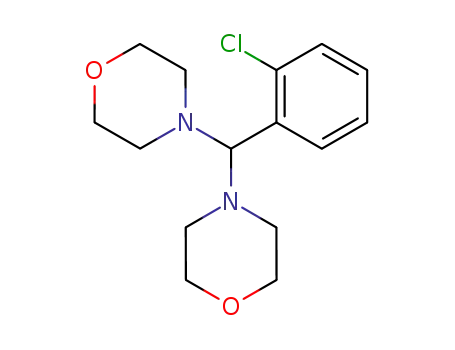
(2-chloro-phenyl)-dimorpholino-methane
