<ol id="33bym"></ol> 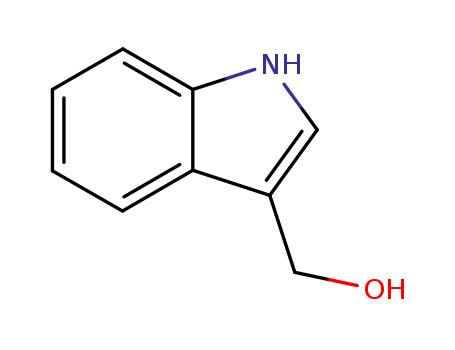

Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Preparation |
Using indole, phosphorus oxychloride and N,N-dimethylformamide as raw materials, indole-3-carbaldehyde was synthesized by Vilsmeier-Haack reaction with a yield of 87.1%. |
|
Synthesis Reference(s) |
Canadian Journal of Chemistry, 31, p. 775, 1953 DOI: 10.1139/v53-106The Journal of Organic Chemistry, 61, p. 1493, 1996 DOI: 10.1021/jo951219c |
|
Biochem/physiol Actions |
Inhibits cancinogenesis at the initiation stage. Has be shown to inhibit carcinogenesis in several animal species, but it enhances tumor incidence if administered at a post-initiation stage. Found in cruciferous vegetables. |
|
Anticancer Research |
I3C is a bioactive compound majorly found in Brassica vegetables including broccoli,cauliflower, and collard greens. I3C and its derivative diindolylmethane (DIM)have been investigated for cancer prevention and treatment of breast, prostate, andovarian cancers. I3C partially modulates the tyrosine kinase/PI3K/Akt signalingpathway which leads to the prevention of lung adenocarcinoma which is induced byusing tobacco carcinogen in A/J mice. DIM transduces signaling via aryl hydrocarbon(Ah) receptor, NF-κB/Wnt/Akt/mTOR signaling pathways, cell cycle arrest,modulated cytochrome P450 enzymes, and altered angiogenetic and invasive,metastatic, and epigenetic behavior of cancer cells. Combination of DIM and I3Cinduces Nrf2-mediated phase II drug metabolizing genes (GSTm2, UGT1A1, andNQO1) and antioxidant genes (HO-1, SOD-1) (Weng et al. 2008; 2012). |
|
Sources |
Indole-3-carbinol (I3C) is a bioactive phytochemical found abundantly in cruciferous vegetables such as broccoli, cabbage, green peas, cauliflower, and Brussels sprouts.[1] |
|
Categories and Type |
I3C is classified as a bioactive phytochemical and belongs to the group of indole compounds. |
|
Mechanism of Action |
I3C and its metabolites alter multiple signaling pathways controlling cellular events such as oxidation, inflammation, proliferation, differentiation, apoptosis, angiogenesis, and immunity. DIM, a metabolite of I3C, is particularly involved in modulating aryl hydrocarbon receptor (AHR) signaling.[3] |
|
Production Methods |
I3C is naturally occurring and is produced when raw cruciferous vegetables are consumed or processed. It can also be obtained through extraction and purification processes for use in dietary supplements or pharmaceuticals. |
|
Definition |
ChEBI: Indole-3-carbinol is an indolyl alcohol carrying a hydroxymethyl group at position 3. It is a constituent of the cruciferous vegetables and had anticancer activity. |
|
Application |
Indole-3-carbinol (I3C) is a secondary plant metabolite of 3-methylindole produced in Brassica family vegetables. It is an inhibitor of Amyloid-beta deposition and Inhibits cancinogenesis at the initiation stage. Indole-3-carbinol is used in cancer prevention, specifically for breast, cervical and colon cancers. It has also been widely used to address lupus. |
|
General Description |
Indole-3-carbinol is a novel secondary plant metabolite produced in cruciferous vegetables, such as cabbage, cauliflower and brussels sprouts. |
InChI:InChI=1/C9H9NO/c11-6-7-5-10-9-4-2-1-3-8(7)9/h1-5,10-11H,6H2
-
A novel triazine-phosphanimine polymeric...
A new surfactant (quercetin) assisted hy...
A series of novel indole derivatives wer...
An unprecedented tandem N-alkylation-ion...
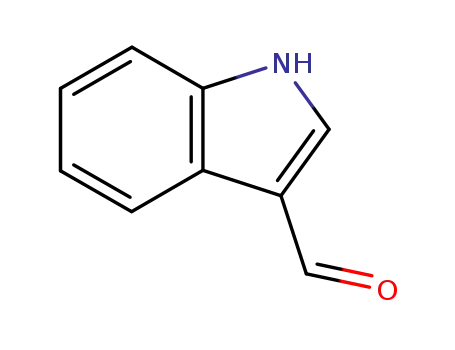
Indole-3-carboxaldehyde

indole-3-carbinol
| Conditions | Yield |
|---|---|
|
With
sodium tetrahydroborate;
In
methanol;
at 0 ℃;
for 1h;
|
100% |
|
With
formic acid;
In
ethanol;
at 80 ℃;
for 12h;
Catalytic behavior;
|
91% |
|
With
ammonia; calcium;
In
tetrahydrofuran;
at -33 ℃;
for 2h;
other reagents: lithium, sodium, liq. ammonia;
|
80% |
|
With
tricobalt tetraoxide;
In
isopropyl alcohol;
at 50 ℃;
for 0.5h;
chemoselective reaction;
Green chemistry;
|
80% |
|
With
sodium tetrahydroborate;
In
methanol;
for 1h;
Ambient temperature;
|
57% |
|
With
ethanol; platinum;
Hydrogenation;
|
|
|
With
sodium borate; ethanol;
|
|
|
With
methanol; sodium borate;
|
|
|
With
1,4-dioxane; sodium borate;
|
|
|
With
tetrahydrofuran; lithium borate;
|
|
|
With
sodium tetrahydroborate;
|
|
|
With
cytochrome P450BM-3 F87A/T268A variant; NADPH;
at 37 ℃;
pH=7.4;
aq. phosphate buffer;
Enzymatic reaction;
|
|
|
With
sodium hydroxide;
In
isopropyl alcohol;
at 82 ℃;
for 2h;
|
88 %Chromat. |
|
With
sodium tetrahydroborate;
In
tetrahydrofuran; ethanol;
at 20 ℃;
for 3h;
|
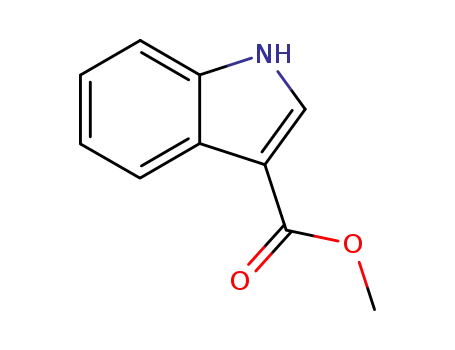
3-methoxycarbonylindole

indole-3-carbinol
| Conditions | Yield |
|---|---|
|
3-methoxycarbonylindole;
With
pentadec-3-en-2-one; trimethylsilyl trifluoromethanesulfonate; triphenylphosphine;
In
dichloromethane;
at -78 - 0 ℃;
for 1h;
Inert atmosphere;
With
diisobutylaluminium hydride;
In
hexane; dichloromethane;
at -78 ℃;
Inert atmosphere;
With
potassium carbonate;
In
methanol; hexane; dichloromethane;
at 20 ℃;
for 0.5h;
Inert atmosphere;
|
84% |
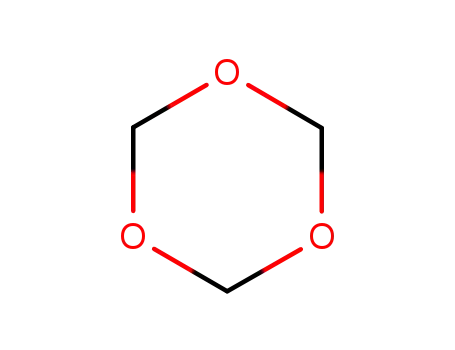
1,3,5-Trioxan
indole
methanol
sodium methylate
N-morpholinyl-3-indolylmethylamine
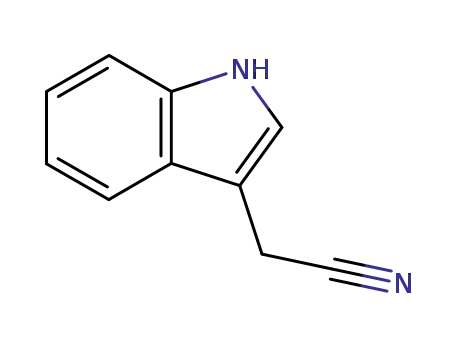
indole-3-acetonitrile
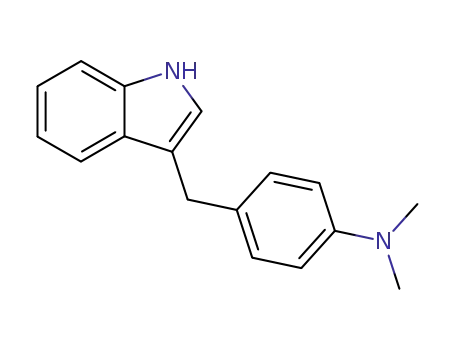
3-((4-(N,N-Dimethylamino)phenyl)methyl)-indole
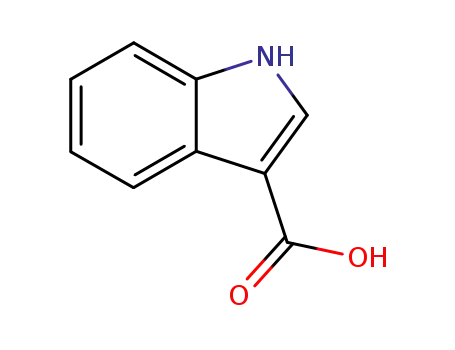
1H-indole-3-carboxylic acid
