<ol id="33bym"></ol> 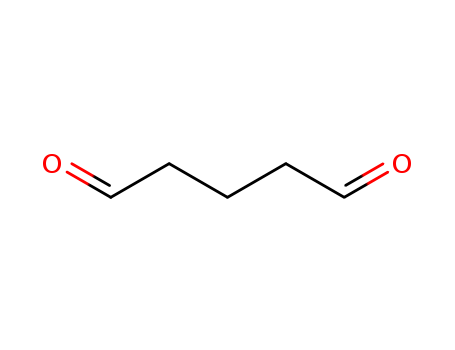

Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Chemical Description |
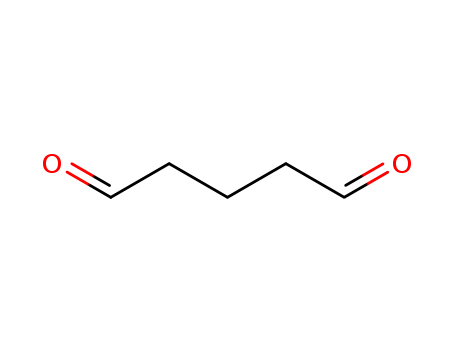
Glutaraldehyde is a C5 dialdehyde and a biomimetic equivalent of l-lysine. |
|
Synthesis Reference(s) |
Tetrahedron, 48, p. 3503, 1992 DOI: 10.1016/S0040-4020(01)88489-1 |
|
Air & Water Reactions |
Polymerizes in the presence of water. |
|
Reactivity Profile |
GLUTARALDEHYDE may discolor on exposure to air. Pentanedial polymerizes on heating. Pentanedial is incompatible with strong oxidizing agents. Pentanedial polymerizes in the presence of water. |
|
Health Hazard |
Glutaraldehyde is a strong irritant to the nose,eyes, and skin. In rabbits, 250 μg and 500 mg in 24 hours produced severe irritation in theeyes and skin, respectively. The corrosiveeffect on human skin of 6 mg over 3 dayswas severe. However, the acute toxicity ofglutaraldehyde by the oral and dermal routesis low to mild. Ohsumi and Kuroki (1988)determined that the symptoms of acute toxicityof this compound were less severethan those of formaldehyde. But the restraintof growth was more pronounced in micetreated with glutaraldehyde. An oral LD50value of 1300 mg/kg was reported for mice.Inhalation of this compound can cause upperrespiratory tract irritation, headache, and nervousness.Mice exposed at 33 ppm showedsymptoms of hepatitis. |
|
Fire Hazard |
Literature sources indicate that Pentanedial is nonflammable. |
|
Flammability and Explosibility |
Nonflammable |
|
Biochem/physiol Actions |
Glutaraldehyde is an effective protein crosslinker and finds application in techniques like enzyme immobilisation microscopy, histochemistry and cytochemistry. It exists in different forms under acidic or neutral conditions. It is a biocide widely used as a disinfectant in hospitals and industries and is toxic to aquatic organisms. Its allergic nature leads to hypersensitivity reactions. Contact of glutaraldehyde vapors in endoscopy contributes to Colitis. |
|
Contact allergens |
Glutaraldehyde is a well-know sensitizer in cleaners and health workers. It can also be found in X-ray developers or in cosmetics. |
|
Safety Profile |
Poison by ingestion, intravenous, and intraperitoneal routes. Moderately toxic by inhalation, skin contact, and subcutaneous routes. Experimental teratogenic and reproductive effects. A severe eye and human skin irritant. Mutation data reported. When heated to decomp osition it emits acrid smoke and irritating fumes. See also ALDEHYDES. |
|
Potential Exposure |
Glutaraldehyde is used in leather tanning; in embalming fluids; as a germicide; as a cross-linking agent for protein and polyhydroxy materi als; as a fixative for tissues; and as an intermediate. Buffered solutions are used as antimicrobial agents in hospitals. |
|
Shipping |
UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. |
|
Purification Methods |
Likely impurities are oxidation products-acids, semialdehydes and polymers. It can be purified by repeated washing with activated charcoal (Norit) followed by vacuum filtration, using 15-20g charcoal/100mL of glutaraldehyde solution. Distil it at 60-65o/15mm, discarding the first 5-10%, then dilute with an equal volume of freshly distilled water at 70-75o, using magnetic stirring under nitrogen. The solution is stored at low temperature (3-4o), in a tightly stoppered container, and protected from light. Standardise by titration with hydroxylamine. [Anderson J Histochem Cytochem 15 652 1967, Beilstein 1 IV 3659.] |
|
Incompatibilities |
Water contact forms a polymer solution. A strong reducing agent. Incompatible with strong acids; caustics, ammonia, amines, and strong oxidizers. Note: Alkaline solutions of glutaraldehyde (i.e., activated glutar aldehyde) react with alcohol, ketones, amines, hydrazines, and proteins. |
|
Waste Disposal |
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. |
|
Definition |
ChEBI: A dialdehyde comprised of pentane with aldehyde functions at C-1 and C-5. |
|
General Description |
Light yellow liquid. Mixes with water. |
InChI:InChI=1/C5H8O2/c6-4-2-1-3-5-7/h4-5H,1-3H2
A hierarchical microporous-mesoporous me...
A series of niobium-containing mesoporou...
12-Heteropoly acids exhibited high perfo...
A highly active and perfectly structured...
We report highly enantioselective exampl...
A novel WO3/SiO2 was designed for the ca...
Metal organic framework Fe-MIL-101-NH2 w...
Reported here are some aspects of the at...
The tandem [4+2]/[3+2] cycloaddition of ...
-
-
Keggin-structured 12-tungstophosphoric a...
A novel and green route is reported for ...
The calcium doped CoFe2O4 spinels CaxCo1...
A novel WO3/SiO2 was prepared by incipie...
The function of ammonium salts on the ep...
The invention relates to a method for ca...
PROBLEM TO BE SOLVED: To provide a proce...
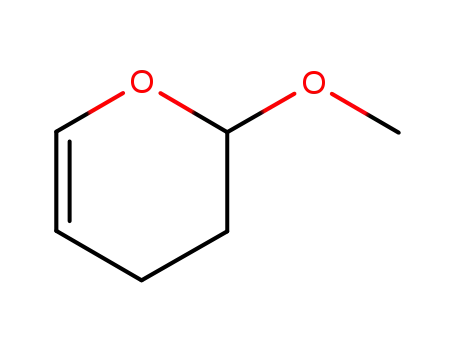
2-methoxy-3,4-dihydro-2H-pyran

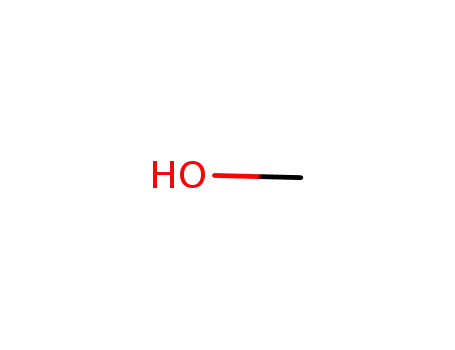
methanol

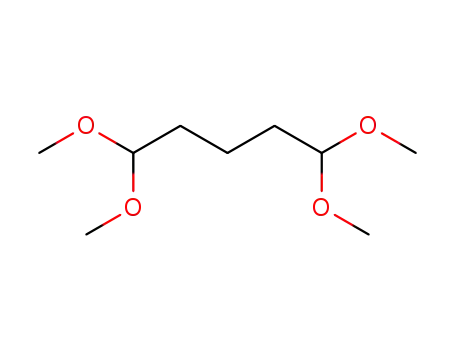
1,1,5,5-tetramethoxy-pentane

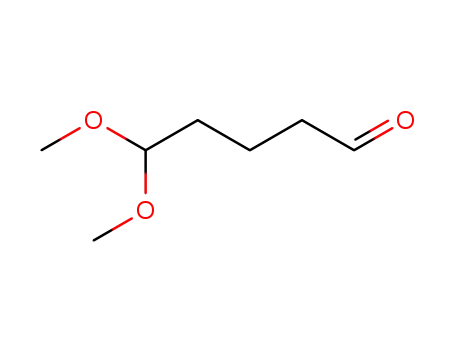
5,5-dimethoxypentanal

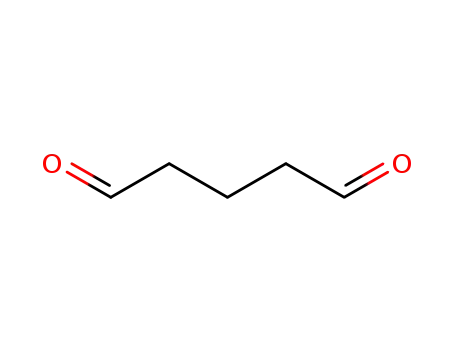
Glutaraldehyde
| Conditions | Yield |
|---|---|
|
With
water;
at 90 - 93 ℃;
|
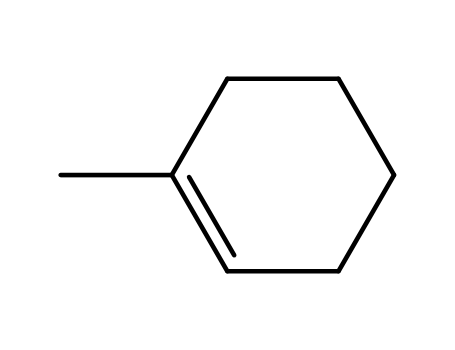
1-methylcyclohex-1-ene

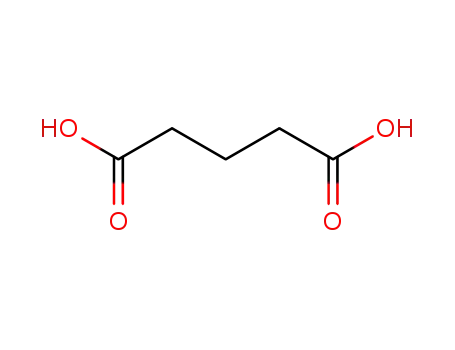
1,5-pentanedioic acid

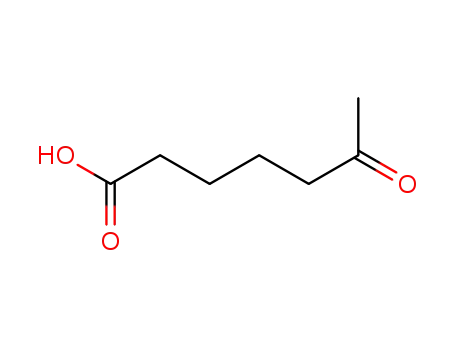
6-oxoheptanoic acid

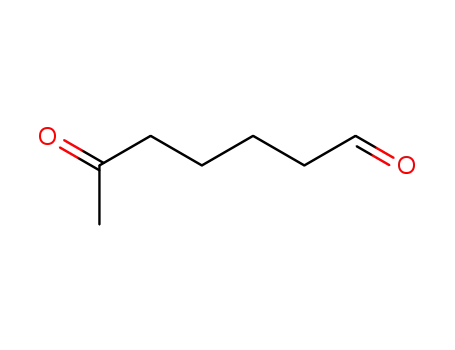
6-oxoheptanal

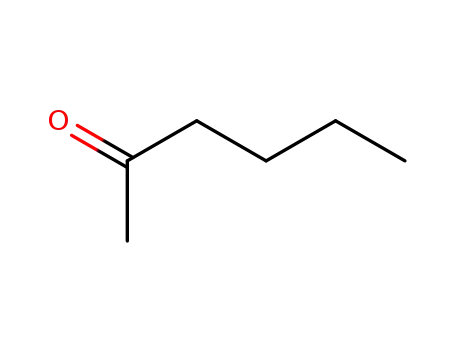
n-hexan-2-one

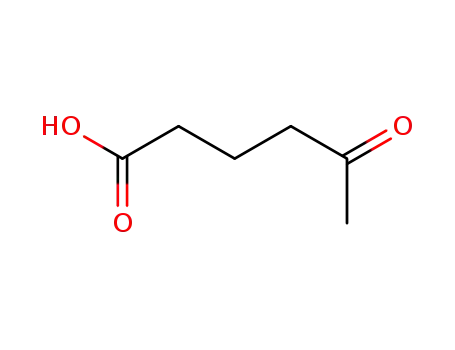
5-ketohexanoic acid


Glutaraldehyde
| Conditions | Yield |
|---|---|
|
With
ozone;
at 30 ℃;
Mechanism;
Product distribution;
multistep reaction;
|
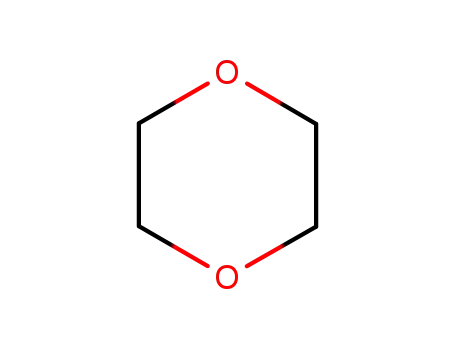
1,4-dioxane
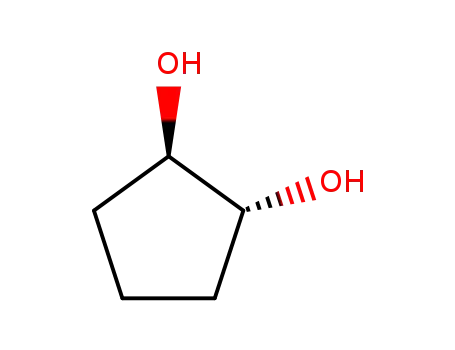
trans-cyclopentane-1,2-diol
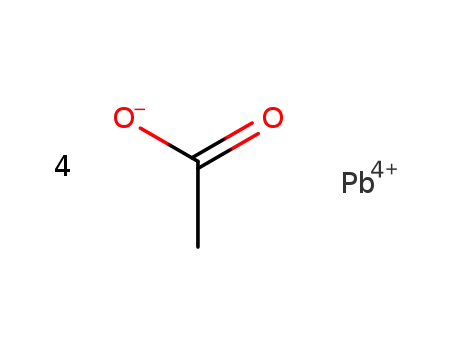
lead(IV) tetraacetate

2-methoxy-3,4-dihydro-2H-pyran
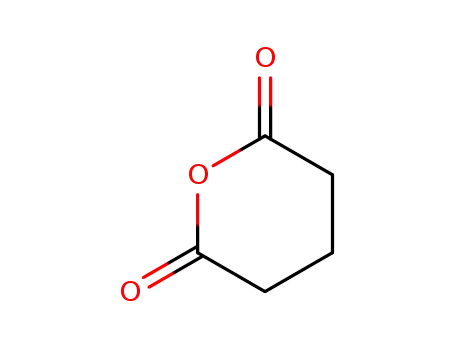
glutaric anhydride,

1,5-pentanedioic acid
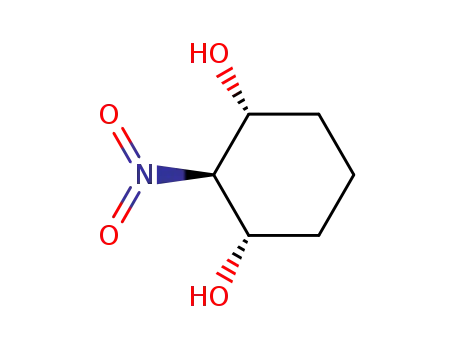
(1α,2β,3α)-2-nitro-1,3-cyclohexanediol
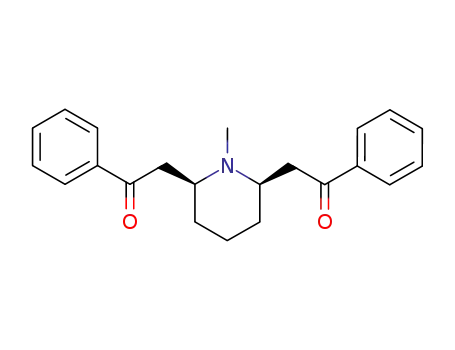
Lobelanine
