
Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Purification Methods |
Crystallise the acid from water and sublime it in a vacuum. Also purify it by dissolving 10g in 100mL of boiling CCl4/CHCl3 (8:2) (1g charcoal), filtering and cooling to 25o. Dry it in vacuo [Kohn et al. Anal Chem 58 3184 1986]. [Beilstein 24 III/IV 1875.] |
|
General Description |
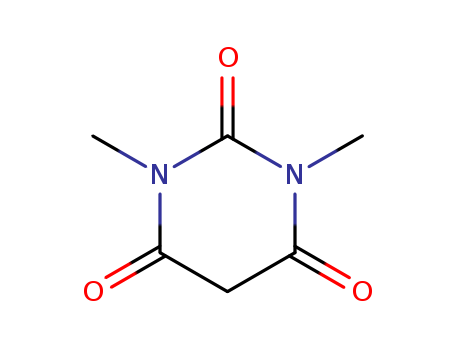
1,3-Dimethylbarbituric acid (1,3-Dimethyl-2,4,6(1H,3H,5H)-pyrimidinetrione) is an active methylene compound. It undergoes hollow Pd6 water-soluble cage, [{(tmen)Pd}6(timb)4](NO3)12 (tmen= N,N,N′,N′-tetramethylethylenediamine, timb=1,3,5-tris(1-imidazolyl)benzene)-catalyzed Knoevenagel condensation reaction with pyrene-1-carboxaldehyde. It undergoes self-sorted Pd7 molecular boat having an internal nanocavity (catalyst)-assisted Knoevenagel condensation reaction with various aromatic aldehydes. It has been synthesized by reacting 1,3-dimethylurea, malonic acid and acetic anhydride in acetic acid. It is widely used for the synthesis of various synthetic intermediates and heterocyclic compounds. |
InChI:InChI=1/C6H8N2O3/c1-7-4(9)3-5(10)8(2)6(7)11/h3H2,1-2H3
Second-order rate constants (k2) of the ...
A series of 5,5′-(arylmethylene)bis[1,3-...
Novel, inexpensive, and relatively exped...
Fast and reversible dynamic covalent C=C...
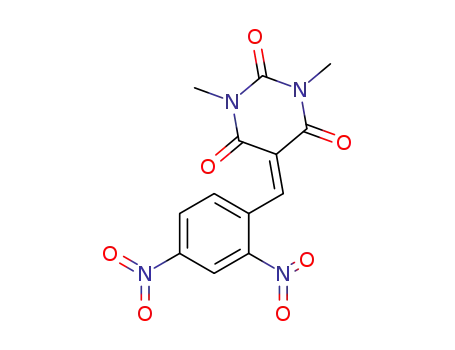
5-(2',4'-dinitrobenzylidene)-1,3-dimethylbarbituric acid

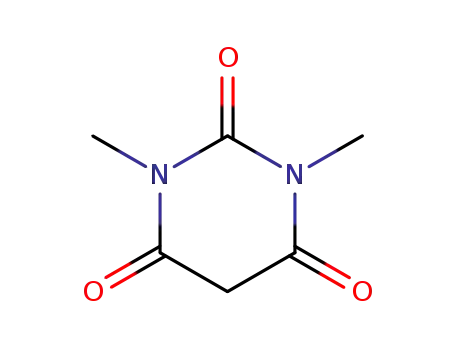
1,3-dimethylbarbituric acid


2,4-dinitrobenzaldehyde
| Conditions | Yield |
|---|---|
|
With sodium chloride; In water; at 25 ℃; Equilibrium constant;
|
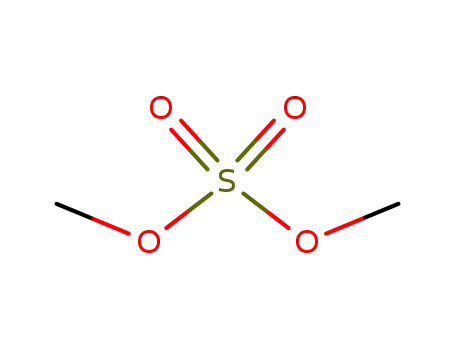
dimethyl sulfate

C5H4N2O3(2-)*2K(1+)


1,3-dimethylbarbituric acid


1,3,5-trimethylbarbituric acid

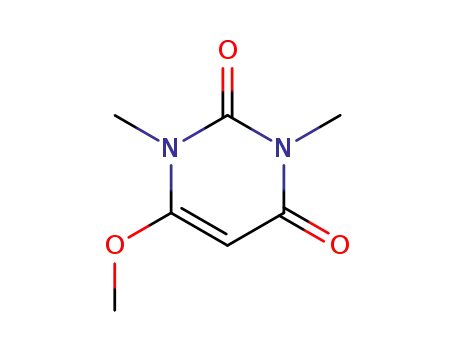
6-methoxy-1,3-dimethyluracil
| Conditions | Yield |
|---|---|
|
In water; at 10 ℃; Yield given. Yields of byproduct given;
|

BARBITURIC ACID

dimethyl sulfate
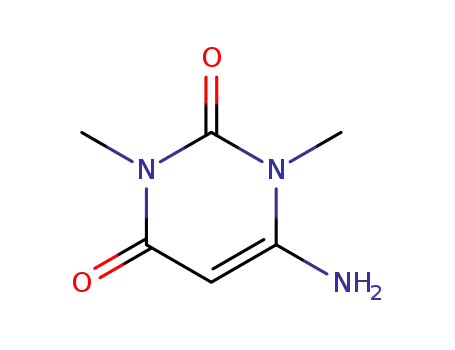
6-Amino-1,3-dimethylbarbituric acid
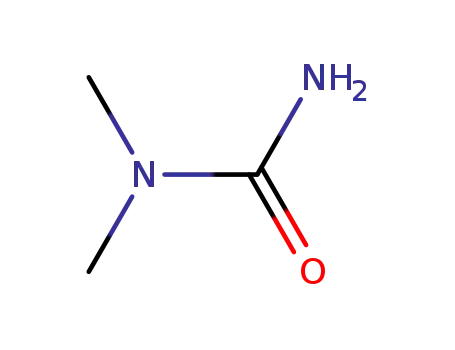
1,1-Dimethylurea

1,3-Dimethyl-5-aminomethylen-barbitursaeure

5-(furan-2-ylmethylene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione
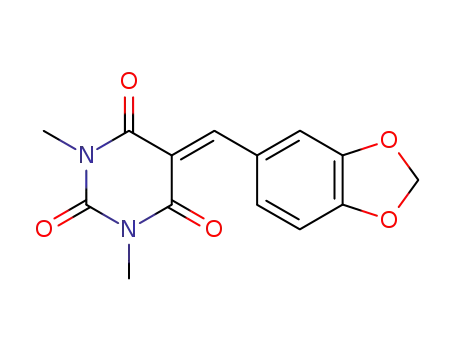
5-(benzo[d][1,3]dioxol-5-ylmethylene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione

1,3,1',3'-tetramethyl-5,5'-methanylylidene-bis-pyrimidine-2,4,6-trione
