<ol id="33bym"></ol> 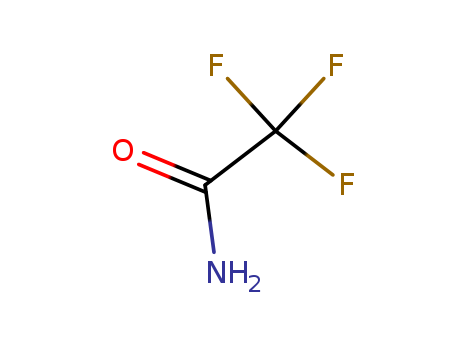

Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Preparation |
One-pot synthetic approach to produce trifluoroacetamide has been developed using an electrochemical method with the B12 complex as a catalyst under mild conditions, in open air at room temperature. Thirty examples of trifluoroacetamide were synthesized from 1,1,1-trichloro-2,2,2-trifluoroethane (CFC-113a) in moderate to good yields. This user-friendly strategy is compatible with a broad range of trifluoroacetamide syntheses. 10.1142/S1088424621500292 |
|
Application |
Trifluoroacetamide was used as probe for determination of membrane potential and extra/intracellular volume of erythrocytes by fluorine-19 NMR studies. |
|
General Description |
Trifluoroacetamide is a good quencher of tryptophan fluorescence. |
InChI:InChI=1/C4ClF10P/c5-16(3(12,13)1(6,7)8)4(14,15)2(9,10)11
The invention discloses a method for pre...
A thermal reaction of amines, anilines, ...
Emerging applications in the field of ch...
The present invention provides systems a...
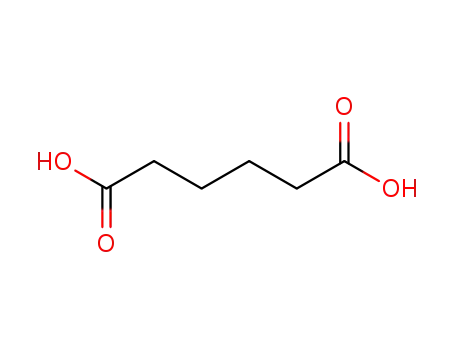
Adipic acid

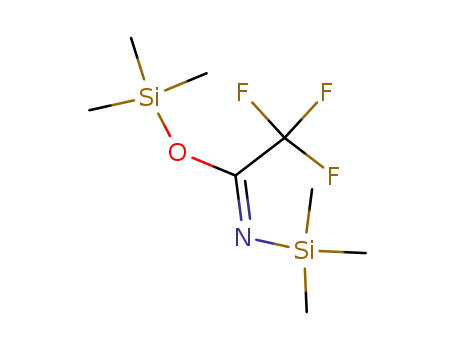
N,O-bis(trimethylsilyl)trifluoroacetamide

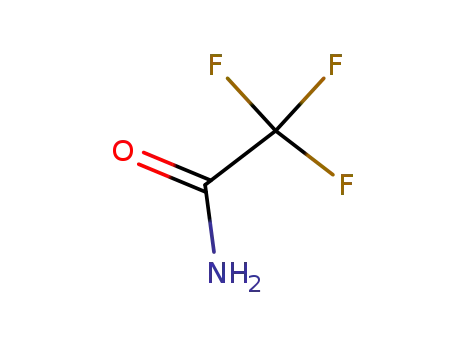
2,2,2-trifluoroacetamide

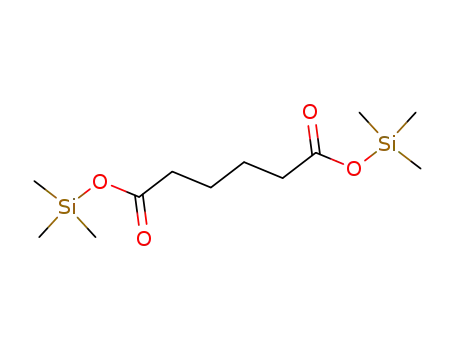
Bis(trimethylsilyl) adipate

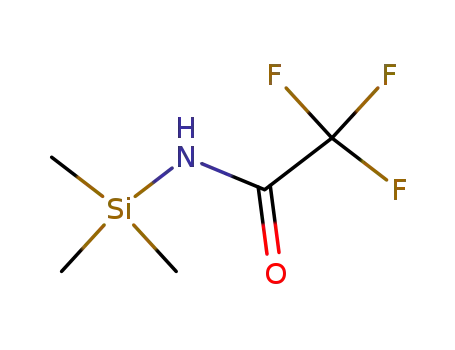
N-(trimethylsilyl)trifluoroacetamide
| Conditions | Yield |
|---|---|
|
In
hexane; dichloromethane;
at 60 ℃;
for 0.666667h;
|
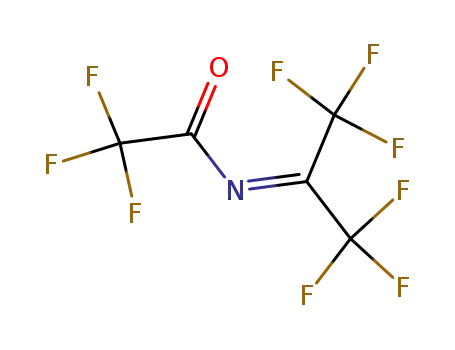
2,2,2-trifluoro-N-{2,2,2-trifluoro-1-(trifluoromethyl) ethylidene}acetamide

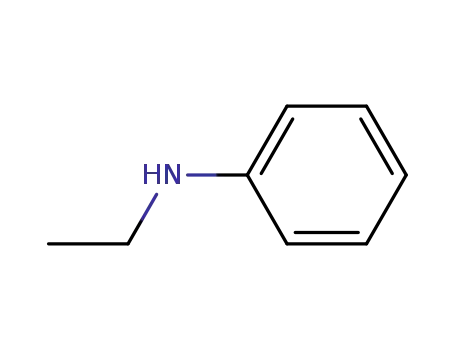
N-ethyl-N-phenylamine

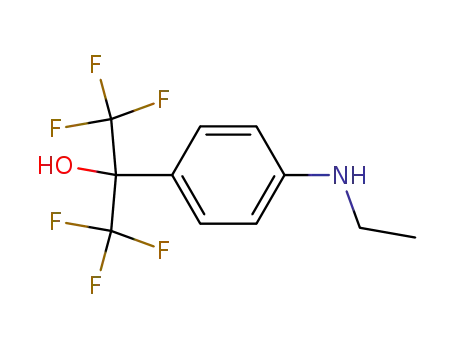
2-(4-ethylamino-phenyl)-1,1,1,3,3,3-hexafluoro-propan-2-ol

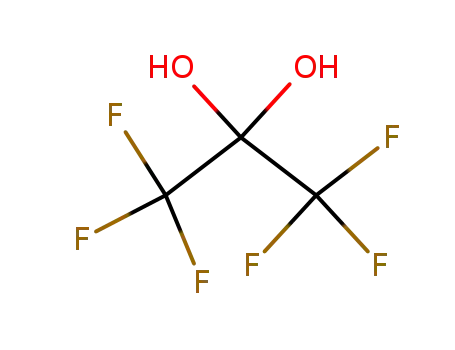
hexafluoroacetone hydrate


2,2,2-trifluoroacetamide

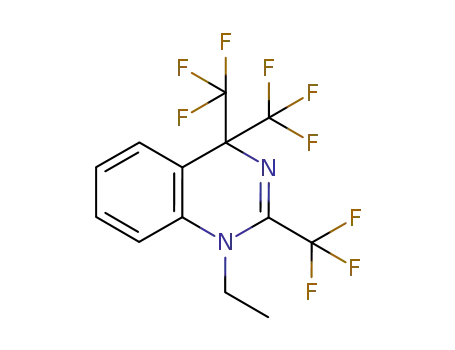
2,4,4-tris(trifluoromethyl)-1-ethyl-1,4-dihydroquinazoline

![N-[1-(Ethyl-phenyl-amino)-2,2,2-trifluoro-1-trifluoromethyl-ethyl]-2,2,2-trifluoro-acetamide](/upload/2025/4/903d3ea4-442d-4b74-8fef-5557dec57fc8.png)
N-[1-(Ethyl-phenyl-amino)-2,2,2-trifluoro-1-trifluoromethyl-ethyl]-2,2,2-trifluoro-acetamide
| Conditions | Yield |
|---|---|
|
In
chloroform;
at 20 ℃;
for 1440h;
Mechanism;
|
87% |
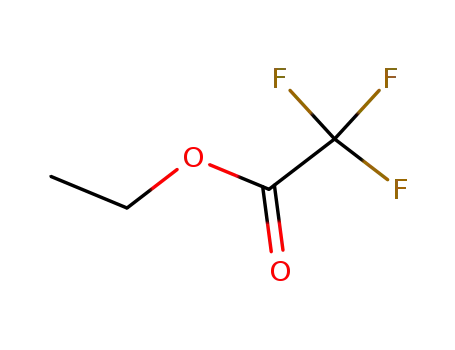
ethyl trifluoroacetate,
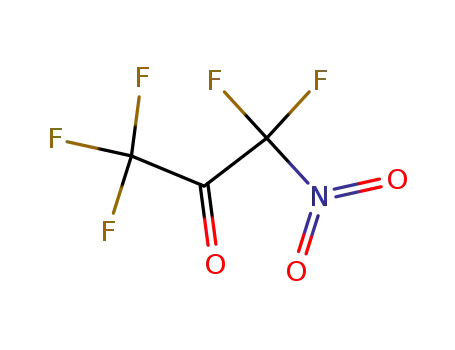
nitropentafluoroacetone
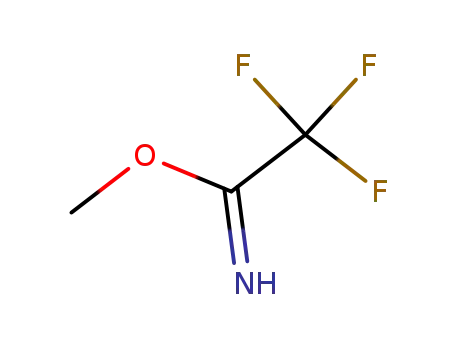
2,2,2-trifluoroacetimidic acid methyl ester

N-(trimethylsilyl)trifluoroacetamide
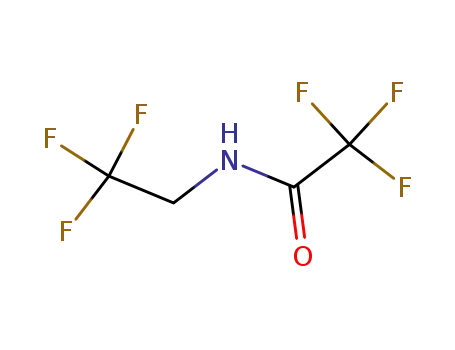
N-(2,2,2-trifluoroethyl)trifluoroacetamide
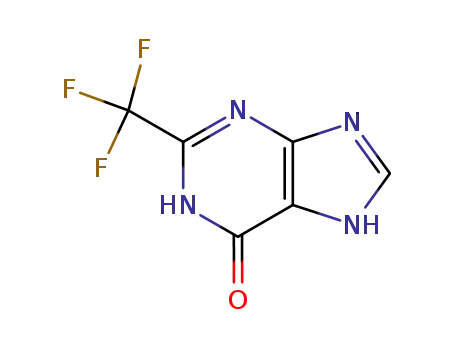
2-trifluoromethyl-1,7-dihydro-purin-6-one
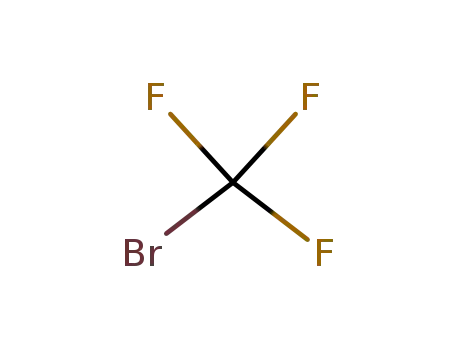
Bromotrifluoromethane
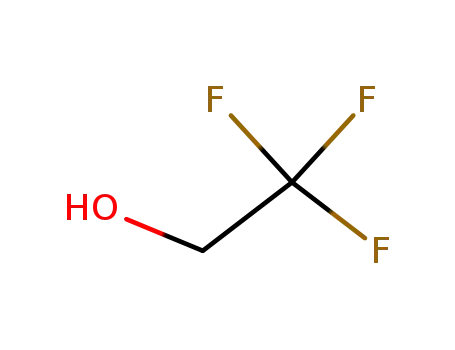
2,2,2-trifluoroethanol
