<ol id="33bym"></ol> 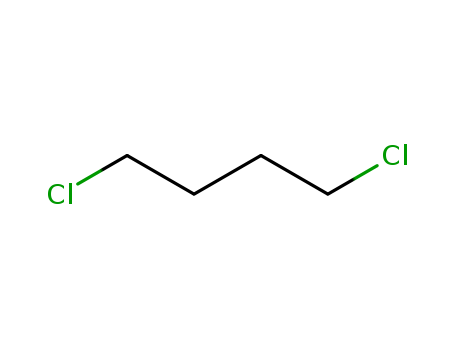

Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
General Description |
1,4-dichlorobutane, also known as 1,4-DCB, is a chemical compound with the formula C4H8Cl2. It is a colorless liquid with a sharp, pungent odor and is insoluble in water but soluble in organic solvents. 1,4-DCB is primarily used as an intermediate in the production of other chemicals, including pharmaceuticals and pesticides. It is also used as a solvent and as a reagent in organic synthesis. The compound is classified as a chlorinated hydrocarbon and is considered to be toxic to the environment, with potential harmful effects on aquatic organisms. It is important to handle and dispose of 1,4-dichlorobutane with care to minimize its impact on human health and the environment. |
InChI:InChI=1/C4H8Cl2/c1-2-3-4(5)6/h4H,2-3H2,1H3
We made use of four methods for determin...
The photochlorinations of the n-butyl, n...
-
-
In contrast to photolysis or thermal dec...
-
The Diels-Alder reactions of trichloroph...
-
The title reactions of HOOC(CH2)nCOOH ac...
Mesoporous TiO2–carbon nanocomposites we...
The invention discloses a preparation me...
The invention discloses a continuous met...
The invention discloses a quinoline deri...
The invention provides a 1,4-dichlorobut...
ethene

1,2-dichloro-ethane

1,2-dichlorobutane

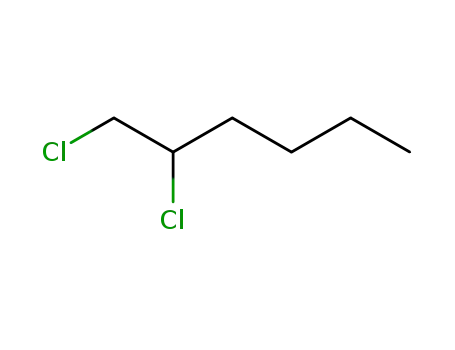
1,2-dichlorohexane

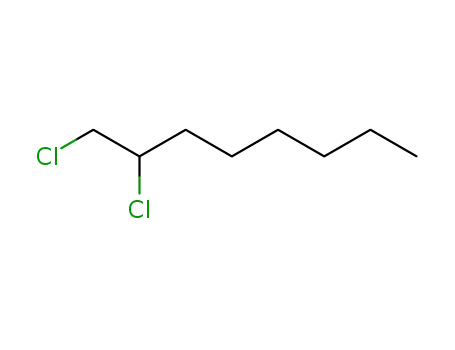
1,2-dichlorooctane

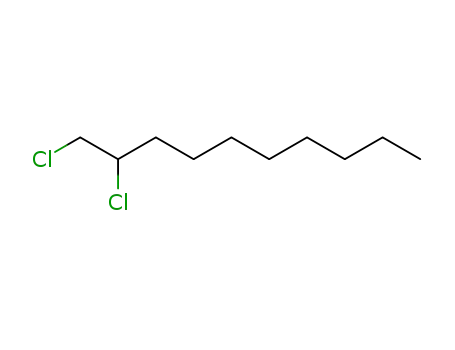
1,2-dichlorodecane

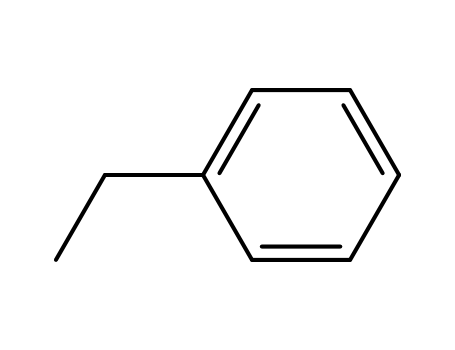
ethylbenzene

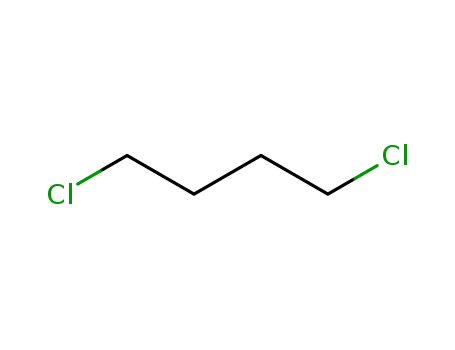
1,4-dichlorobutane
| Conditions | Yield |
|---|---|
|
With
dibenzoyl peroxide;
at 100 ℃;
Product distribution;
Mechanism;
|
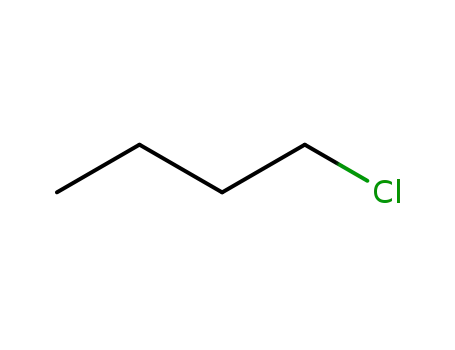
n-Butyl chloride

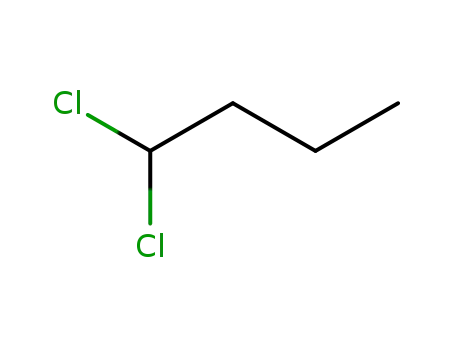
1,1-dichlorobutane
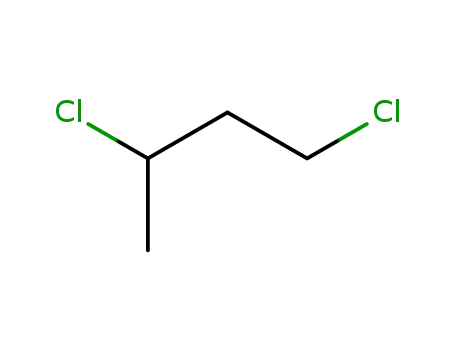
1,3-dichlorobutane


1,2-dichlorobutane


1,4-dichlorobutane
| Conditions | Yield |
|---|---|
|
With
sulfuryl dichloride;
zeolite NaX;
for 2h;
Heating;
Irradiation;
|
6 % Chromat. 23 % Chromat. 46 % Chromat. 25 % Chromat. |
|
With
sulfuryl dichloride;
zeolite NaX;
for 2h;
Product distribution;
Heating;
Irradiation;
|
6 % Chromat. 23 % Chromat. 46 % Chromat. 25 % Chromat. |
|
With
norborn-2-ene; N-chlorohexamethyldisilazane; trans-di-O-tert-butyl hyponitrite;
at 44.9 ℃;
for 1h;
Product distribution;
Mechanism;
further reagent, time;
|
|
|
With
chlorine;
In
tetrachloromethane;
at 20 ℃;
Product distribution;
other solvents, other temperatures;
|
|
|
With
phenylchloroiodonium chloride;
In
tetrachloromethane;
at 30 ℃;
Product distribution;
Mechanism;
Irradiation;
relative rate const., the correlation analysis, subst. phenylchloroiodonium chloride as reagents;
|
|
|
With
N-chloropiperidine; 2,2'-azobis(isobutyronitrile);
In
trifluoroacetic acid;
at 30 ℃;
Product distribution;
Rate constant;
Irradiation;
relative rate constants, position selectivity, substrate selectivity;
|
|
|
With
chlorine;
In
benzene;
at -10.1 ℃;
Product distribution;
dependence of the selectivity of chlorination on the conc. of benzene;
|
|
|
With
N-chloropiperidine; 2,2'-azobis(isobutyronitrile);
In
trifluoroacetic acid;
at 30 ℃;
for 3h;
Product distribution;
Rate constant;
Irradiation;
other objects - relative reactivities and relative rate constants for the various positions of chlorination;
|
|
|
With
chlorine;
In
1,2-dichloro-benzene;
at -20 - 50 ℃;
Product distribution;
Mechanism;
Ratio of products and rate constants; different concentrations of o-dichlorobenzene.;
|
|
|
With
chlorine;
|
|
|
With
sulfuryl dichloride; dibenzoyl peroxide;
|
tetrahydrofuran
Dichloromethylsilane
phosgene
(E)-3-Ureido-but-2-enoic acid ethyl ester
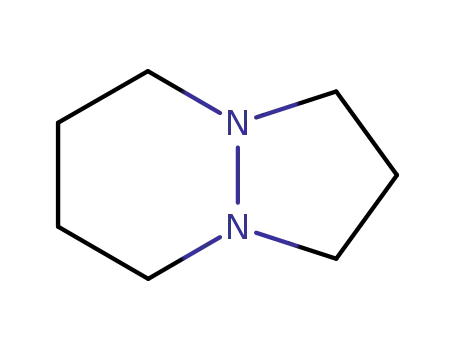
hexahydro-pyrazolo[1,2-a]pyridazine
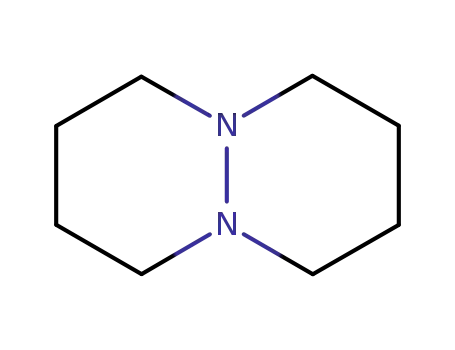
octahydro-pyridazino[1,2-a]pyridazine
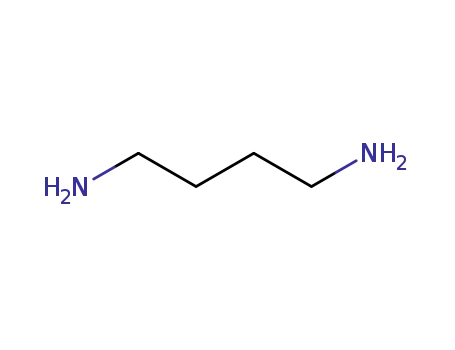
1,4-diaminobutane
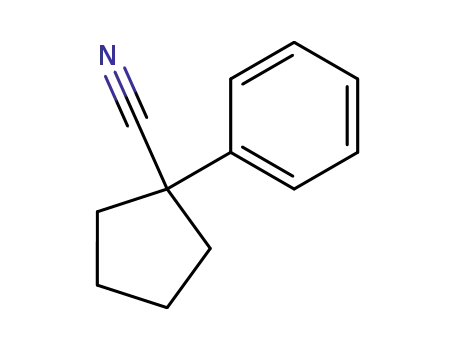
1-phenyl-1-cyclopentanecarbonitrile
