<ol id="33bym"></ol> 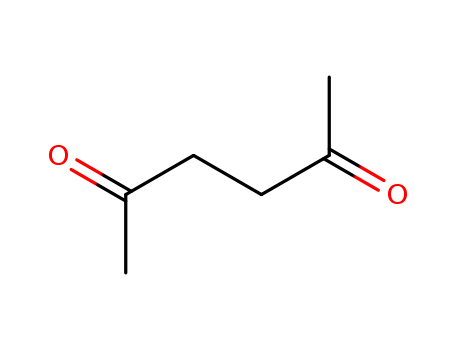

Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Synthesis |
2,5-Hexanedione has been prepared in several ways. A common method involves hydrolysis of 2,5-dimethylfuran, a glucose derived heterocycle. |
|
Mechanism of Toxicity |
Identification of 2,5-hexanedione as the major neurotoxic metabolite of n-hexane proceeded rapidly after its discovery as a urinary metabolite. 2,5-Hexanedione has been found to produce a polyneuropathy indistinguishable from n-hexane. 2,5-Hexanedione is many times more potent than n-hexane, the parent compound, in causing neurotoxicity in experimental animals. It appears that the neurotoxicity of 2,5-hexanedione resides in its γ-diketone structure since 2,3-, 2,4-hexanedione and 2,6-heptanedione are not neurotoxic, while 2,5-heptanedione and 3,6-octanedione and other γ-diketones are neurotoxic. |
|
Synthesis Reference(s) |
Canadian Journal of Chemistry, 59, p. 945, 1981 DOI: 10.1139/v81-137Journal of the American Chemical Society, 105, p. 7200, 1983 DOI: 10.1021/ja00362a047Tetrahedron Letters, 15, p. 4149, 1974 |
|
Air & Water Reactions |
Highly flammable. Water soluble. |
|
Reactivity Profile |
Acetonylacetone is incompatible with oxidizing agents. Acetonylacetone is also incompatible with strong bases and strong reducing agents. |
|
Fire Hazard |
Acetonylacetone is combustible. |
|
Purification Methods |
Purify it by dissolving in Et2O, stiring with K2CO3 (a quarter of the weight of dione), filtering, drying over anhydrous Na2SO4 (not CaCl2), filtering again, evaporating the filtrate and distilling it in a vacuum. It is then redistilled through a 30cm Vigreux column (p 11, oil bath temperature 150o). It is miscible with H2O and EtOH. The dioxime has m 137o (plates from *C6H6), the mono-oxime has b 130o/11mm, and the 2,4-dinitrophenylhydrazone has m 210-212o (red needles from EtOH). It forms complexes with many metals. [Werner et al. Chem Ber 22 2100 1989, for enol content see Gero J Org Chem 19 1960 1954, Beilstein 1 IV 3688.] |
|
Definition |
ChEBI: A diketone that is hexane substituted by oxo groups at positions 2 and 5. It is a toxic metabolite of hexane and of 2-hexanone |
|
General Description |
Clear colorless to amber liquid with a sweet aromatic odor. |
InChI:InChI=1/C6H10O2/c1-5(7)3-4-6(2)8/h3-4H2,1-2H3
A three-step strategy is proposed for th...
-
An organolithium reagent derived form tr...
Dehydrogenation to the corresponding dik...
1,4-Diketones may conveniently be synthe...
5-Hydroxymethylfurfural, a product from ...
Lignocellulosic biomass is an attractive...
-
Dimethyldioxirane was used to monooxide ...
-
-
Methylcyclopentadiene dimer and trimer b...
Zirconium-tungsten mixed oxides (ZrW) ar...
-
Alkynediols isomerized under the catalys...
In the presence of Pd(0) complexes, 2-et...
2,6-Dinitroalkanes have been cyclised to...
Irradiation of ethyl acetoacetate in wat...
Levulinic acid 1 is easily converted by ...
-
It was found for the first time that the...
The transformation of 2,5-dialkylfurans ...
2,5-Hexanedione (HD), which can be produ...
Photocatalytic organic syntheses are oft...
Chemical oscillations in reacting system...
In the reaction of formalin with the 2,5...
A novel approach for the production of 2...
We demonstrated photocatalytic hydrogena...
A series of renewable C9–C12triketones w...
Transient enols including those of aceta...
The α-methyldiphenylsilyl derivatives of...
The Michael addition of primary aliphati...
The kinetics of hydrolysis of 2,5-dimeth...
Although the development of metal nanopa...
A new method for one-step synthesis of k...
-
-
Alcohol dehydrogenase-catalyzed reductio...
Efficient tandem catalytic transformatio...
1H-Pyrroles were converted into 1,4-dica...
Bio-derived furans such as 2-furfural (f...
-
The enantiopure (2S,5S)-hexanediol serve...
Solutions of sulfur dioxide and N-ethyld...
The rates of the reactions, (CH3)2CO -> ...
The invention discloses a method for pre...
Electrochemical cells and photoelectroch...
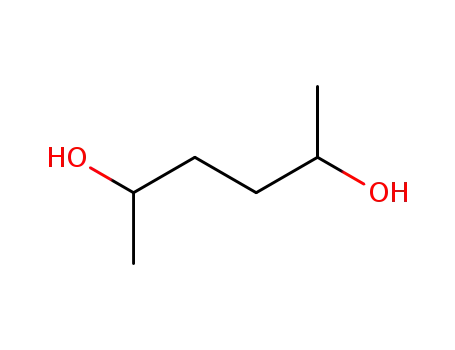
hexane-2,5-diol

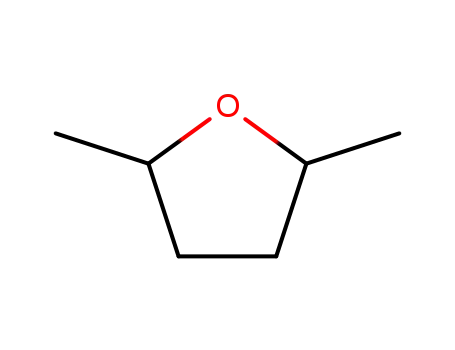
2,5-dimethyltetrahydrofuran

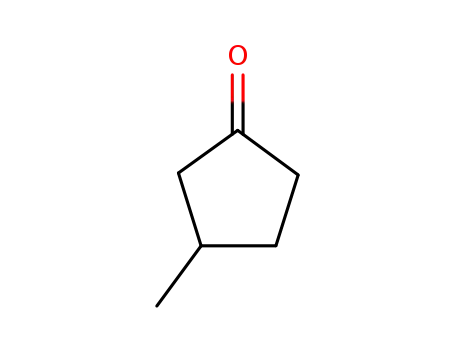
3-methyl-cyclopentanone

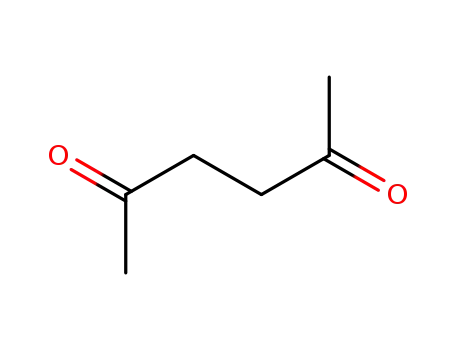
2,5-hexanedione
| Conditions | Yield |
|---|---|
|
at 200 ℃;
|

hexane-2,5-diol


2,5-dimethyltetrahydrofuran


2,5-hexanedione
| Conditions | Yield |
|---|---|
|
With
tetrachloromethane;
bis(acetylacetonate)oxovanadium;
at 100 ℃;
for 1h;
Title compound not separated from byproducts;
|
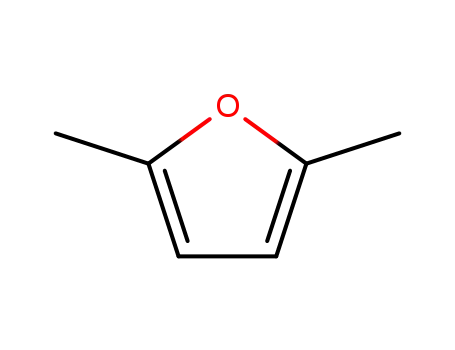
2,5-dimethylfuran
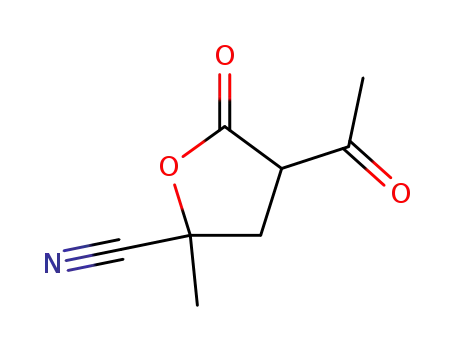
4-acetyl-2-methyl-5-oxo-tetrahydro-furan-2-carbonitrile
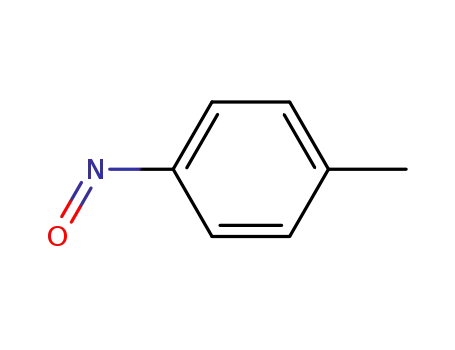
1-methyl-4-nitrosobenzene
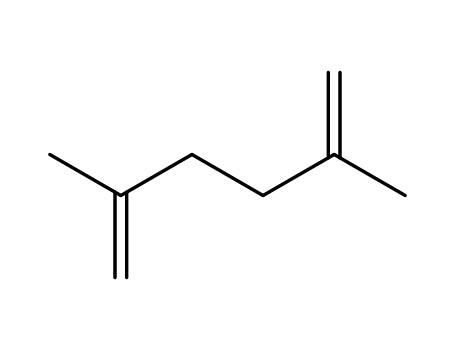
2,5-dimethyl-1,5-hexadiene
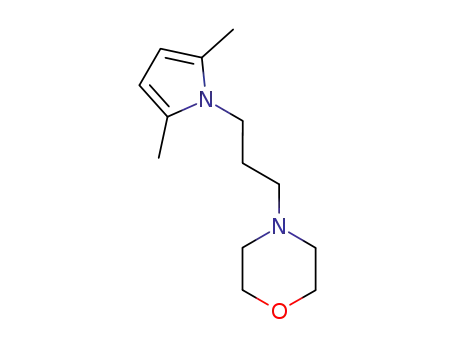
4-[3-(2,5-dimethyl-pyrrol-1-yl)-propyl]-morpholine
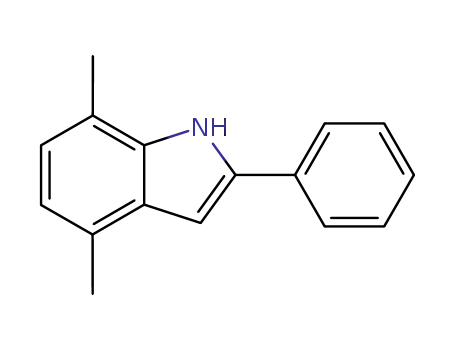
4,7-dimethyl-2-phenyl-indole
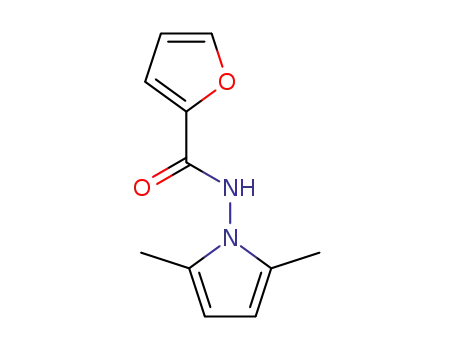
N-(2,5-dimethyl-1H-pyrrol-1-yl)furan-2-carboxamide
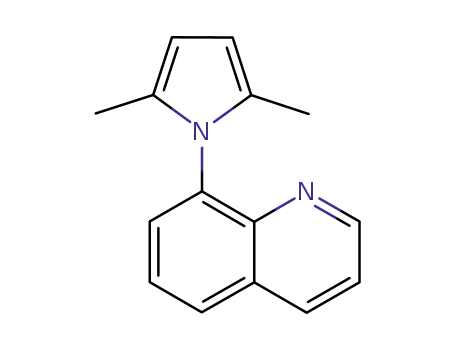
8-(2,5-dimethyl-1H-pyrrol-1-yl)quinoline
