<ol id="33bym"></ol> 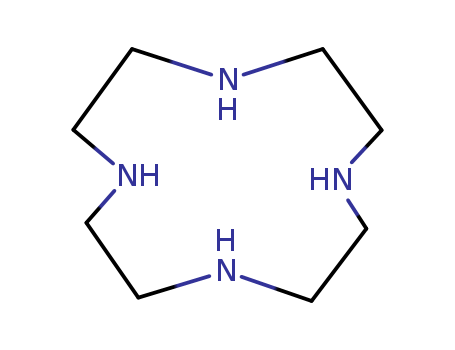

Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 96, p. 2268, 1974 DOI: 10.1021/ja00814a056 |
|
Flammability and Explosibility |
Notclassified |
|
General Description |
Cyclen is a microcyclic tetramine that can be used as a ligand that forms a co-ordination linkage with the surface metal cations. It can be used as a synthetic precursor. It can be prepared by S-alkylation of dithiooxamide with an excess amount of bromoethane. |
InChI:InChI=1/C8H20N4/c1-2-10-5-6-12-8-7-11-4-3-9-1/h9-12H,1-8H2/p+4
Owing to its favorable decay characteris...
The reaction between Cu(NO3)2?3H2O and a...
The 13C and 1H NMR spectra of the comple...
-
-
A practical preparation of the versatile...
The enthalpies of formation of nickel co...
This report outlines a new and efficient...
To provide a metal complex that has high...
The invention discloses a preparation me...
The invention provides a preparation met...
The invention provides a preparation met...
C14H24N4

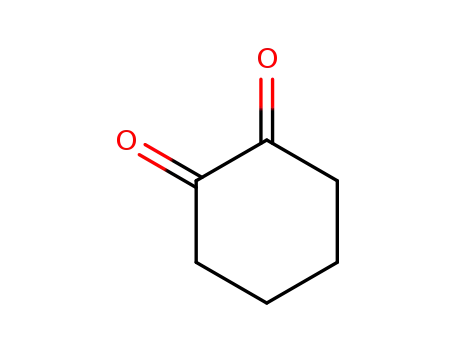
cyclohexane-1,2-dione

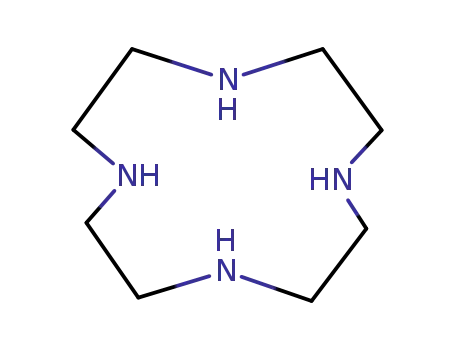
1,4,7,10-tetraazacyclododecan
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
water;
at 60 ℃;
for 6h;
|
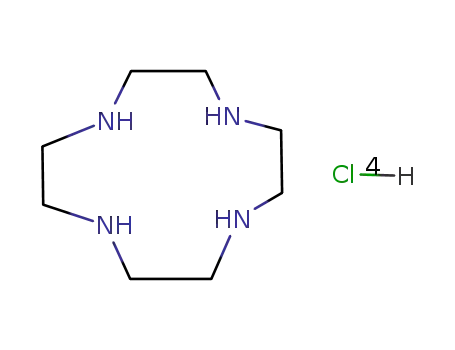
1,4,7,10-tetraazacyclododecane tetrahydrochloride


1,4,7,10-tetraazacyclododecan
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
|
95% |
|
With
potassium hydroxide;
In
water;
at 0 - 10 ℃;
|
91.3% |
|
With
potassium hydroxide;
|
|
|
With
sodium hydroxide;
In
toluene;
at 40 ℃;
for 1h;
Reflux;
|
13.77 g |
|
With
sodium hydroxide;
In
water; toluene;
|
12 g |

1,4,7,10-tetraazacyclododecane tetrahydrochloride
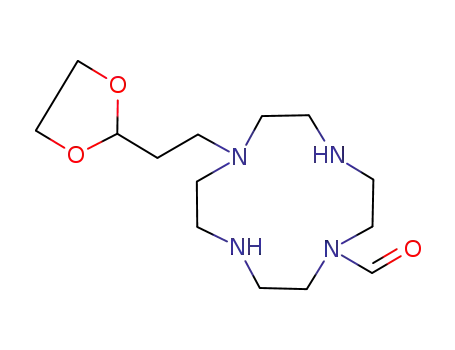
7-<2-(1,3-Dioxolan-2-yl)ethyl>-1,4,7,10-tetraazacyclododecane-1-carbaldehyde
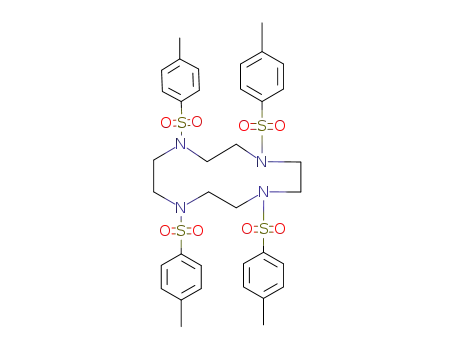
cyclen tetratosylate
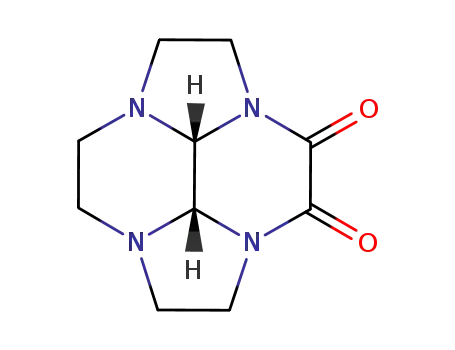
cis-octahydro-2a,4a,6a,8a-tetraazacyclopenta[fg]acenaphthylene-3,4-dione
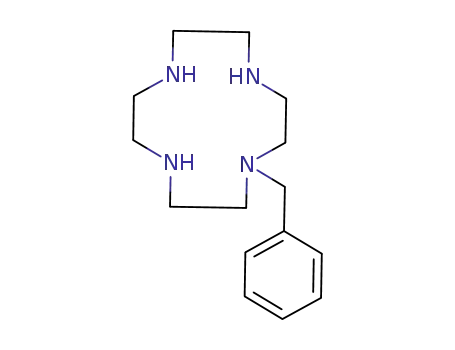
1-benzyl-1,4,7,10-tetraazacyclododecane
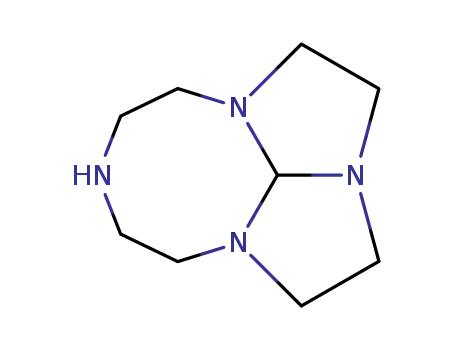
1,4,7,10-tetrazatricyclo[5.5.1.0]tridecane
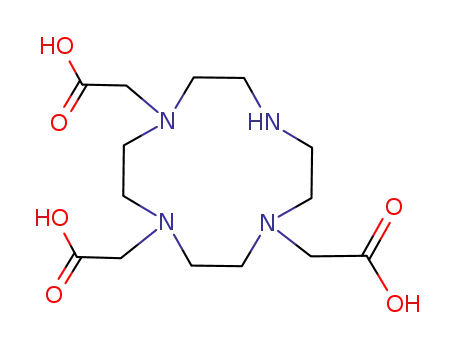
1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid
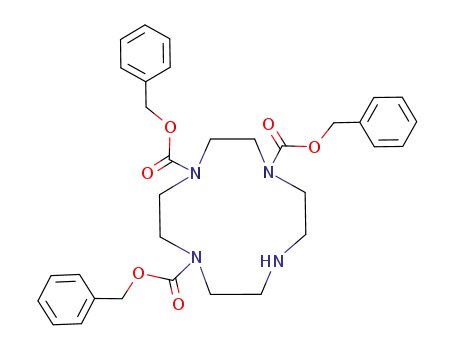
1,4,7-tribenzyl 1,4,7,10-tetraazacyclododecane 1,4,7-tricarboxylate
