<ol id="33bym"></ol> 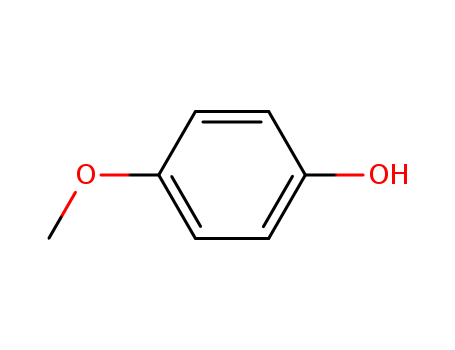

Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Synthesis |
4-Methoxyphenol was synthesised according to Oxidation with H2O2 and a Diselenide catalyst.p-Anisaldehyde (50 mmol) is dissolved in CH2Cl2 (100mL) and (o-NO2PhSe)2 (2 mmol) and 30% H2O2 (13mL, 128 mmol) are added. The mixture is stirred magnetically at room temperature (water bath) for 30 minutes. Insoluble catalyst is removed by filtration and washed with CH2Cl2 (20mL) and water (20mL). It can be reused after drying. To the filtrate and washings, water (100mL) is added, and the layers are separated after shaking. The organic layer is washed subsequently with 10% NaHSO3 (100mL), 10% Na2CO3 (100mL), water (100mL) and dried over Na2SO4. 4-methoxyphenol is obtained by alkaline hydrolysis of the residue. Yield: 93%. |
|
Indications |
Mequinol (4-hydroxyanisole) is a substrate of the enzyme tyrosinase and acts as a competitive inhibitor of melanogenesis. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 42, p. 1479, 1977 DOI: 10.1021/jo00428a054Synthesis, p. 751, 1983 DOI: 10.1055/s-1983-30501Tetrahedron Letters, 34, p. 7667, 1993 DOI: 10.1016/S0040-4039(00)61534-4 |
|
Air & Water Reactions |
Sensitive to moisture. Water soluble. |
|
Reactivity Profile |
4-Methoxyphenol can react with oxidizing materials. |
|
Hazard |
Eye irritant and skin damage. |
|
Health Hazard |
4-Methoxyphenol is expected to cause liver and renal toxicity with narcosis, but only at high levels of exposure. |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
Poison by intraperitoneal route. A skin irritant. When heated to decomposition it emits acrid smoke and fumes. See also ETHERS. |
|
Shipping |
UN3335 Aviation regulated solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required. |
|
Purification Methods |
Crystallise 4-methoxyphenol from *benzene, pet ether or H2O, and dry it under vacuum over P2O5 at room temperature. Sublime it in vacuo. [Wolfenden et al. J Am Chem Soc 109 463 1987, Beilstein 6 IV 5717.] |
|
Chemical Composition and Structure |
Mequinol, also known as 4-methoxyphenol, has the molecular formula C7H8O2. It consists of a phenolic ring with a methoxy group (-OCH3) attached to the aromatic ring at the para position. |
|
Categories and Type |
Mequinol is categorized as a phenolic compound.[1] |
|
Medical Uses |
Mequinol has been incorporated into wound dressing materials due to its anti-inflammatory and antioxidant properties. It has shown potential in promoting wound healing, particularly in diabetic wounds.[2] |
|
Cosmetic Uses |
Mequinol is used in skincare products for its ability to inhibit melanogenesis and treat hyperpigmentation. |
|
Chemical Synthesis |
Mequinol is utilized as an intermediate in the synthesis of various organic compounds, including polymerization inhibitors, antioxidants for foods and cosmetics, and pharmaceuticals.[3] |
|
General Description |
Pink crystals or white waxy solid. |
InChI:InChI=1/C7H8O2/c8-6-9-7-4-2-1-3-5-7/h1-5,8H,6H2
Rate constants for neutral hydrolysis of...
The water-catalyzed hydrolysis of p-meth...
Rate constants are reported for the pH-i...
1-Aryl-3-aryloxymethyl-3-methyltriazenes...
Photolysis and thermolysis of 3,3,6,6-te...
In this work, we report oxidative cleava...
Microwave irradiation at different frequ...
Isobaric thermodynamic activation parame...
A mechanistic investigation of Ullmann-G...
Aryldiazonium tetrafluoroborates, substi...
Biotransformation by resting cultures of...
Both enantiomers of 2-hydroxy-1-phenyl-1...
Amino-Claisen rearrangement of 4-(2-viny...
Bovine serum albumin (BSA) added to the ...
Kinetic solvent effects of N-alkyl-2-pyr...
Upon coordination to {TpW(PMe3)(NO)}, ph...
-
The hydrolysis of N-ethyl O-p-methoxyphe...
Key Word Index - Rosa rugosa; Rosaceae; ...
The photochemical behaviour of selected ...
-
Phosphines are the broadest and most imp...
-
Sulfur-, oxygen-, and phosphorus-substit...
The direct conversion of aryl halides to...
Zn-doped cuprous oxide (Cu2O) nanopartic...
We report the synthesis and structural c...
Synthesis of covalent organic frameworks...
Different carboxy-functionalized imidazo...
Covalent organic frameworks (COFs) have ...
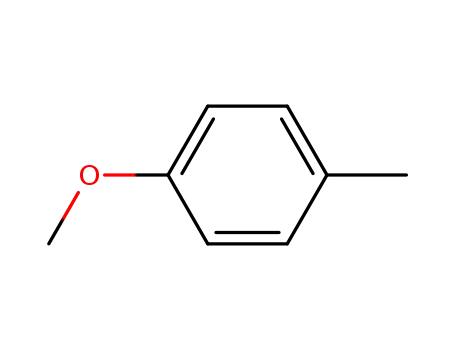
p-methylanizole

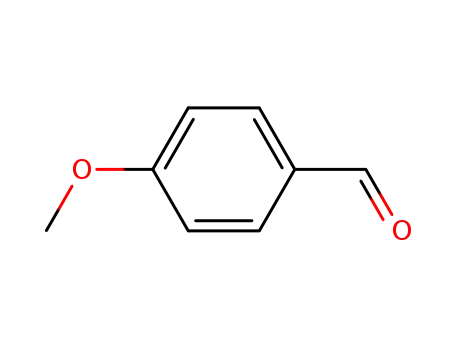
4-methoxy-benzaldehyde

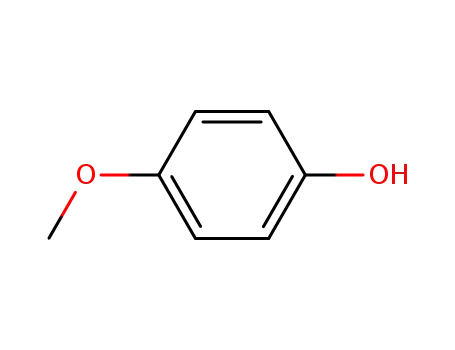
4-methoxy-phenol

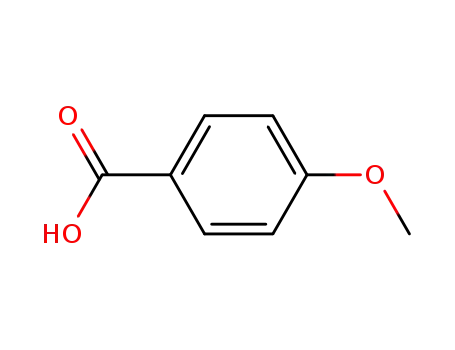
4-methoxybenzoic acid

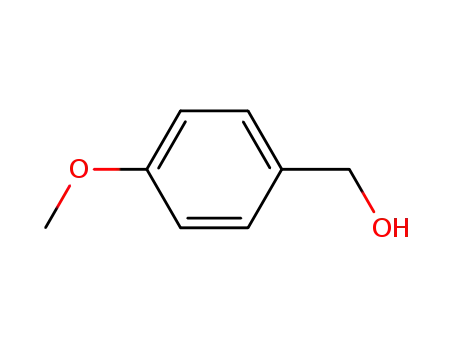
4-Methoxybenzyl alcohol
| Conditions | Yield |
|---|---|
|
With
water; iron; trifluoroacetic acid;
In
pyridine;
for 1h;
Rate constant;
|
|
|
With
water; iron; trifluoroacetic acid;
In
acetone;
for 1h;
Rate constant;
|
|
|
With
water; copper; trifluoroacetic acid;
In
pyridine;
for 1h;
Rate constant;
|
|
|
With
water; copper; trifluoroacetic acid;
In
acetone;
for 1h;
Rate constant;
|
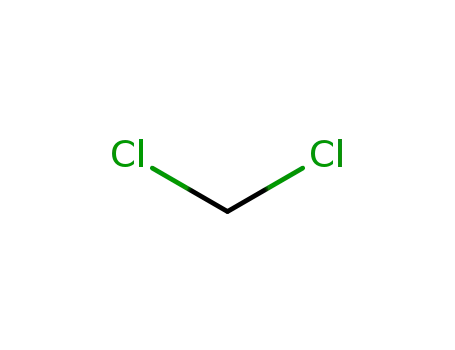
dichloromethane

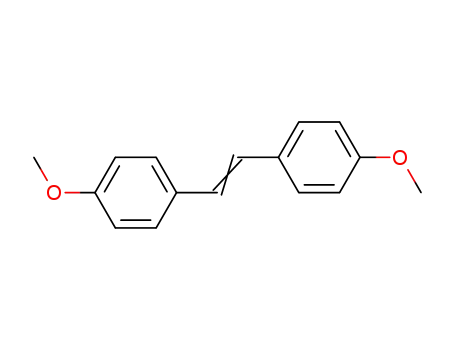
4,4'-dimethoxystilbene


4-methoxy-benzaldehyde


4-methoxy-phenol


4-methoxybenzoic acid
| Conditions | Yield |
|---|---|
|
With
oxygen; ozone;
Yield given. Multistep reaction;
2.) methanol;
|
10 % Chromat. 0.12 g |
|
With
oxygen; ozone;
Yield given. Multistep reaction;
2.) methanol;
|
90 % Chromat. 0.12 g |
|
With
oxygen; ozone;
Yield given. Multistep reaction;
2.) methanol;
|
90 % Chromat. 10 % Chromat. |
tetrahydrofuran
n-butyl magnesium bromide
phosgene
1-methoxy-4-phenoxy-benzene
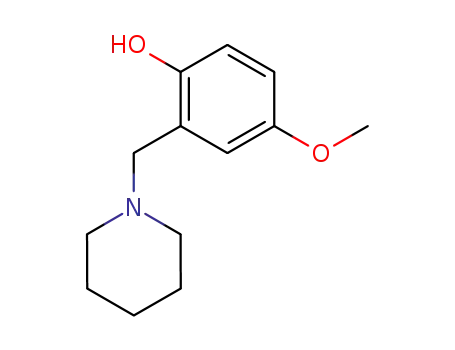
4-methoxy-2-(piperidin-1-ylmethyl)phenol
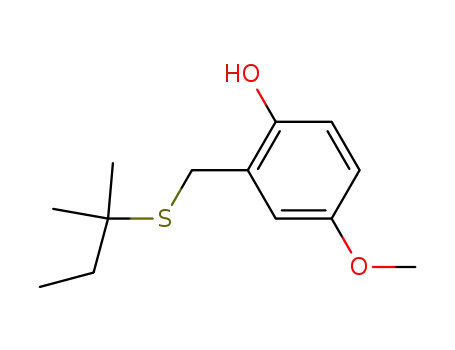
4-methoxy-2-(tert-pentylsulfanyl-methyl)-phenol
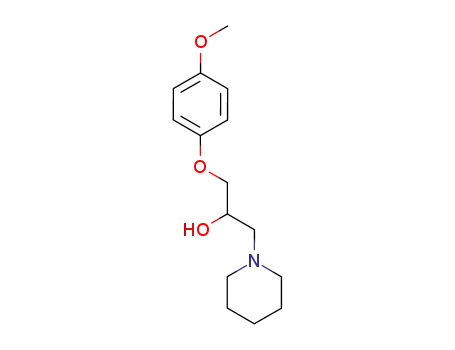
1-(4-methoxy-phenoxy)-3-piperidin-1-yl-propan-2-ol
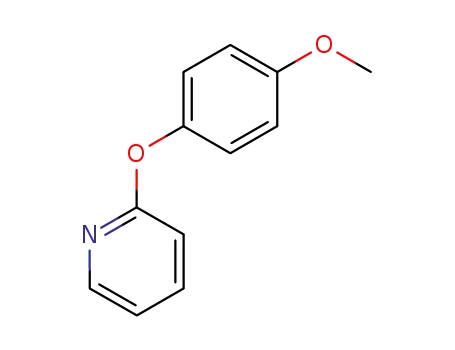
2-(4-methoxyphenoxy)pyridine
