<ol id="33bym"></ol> 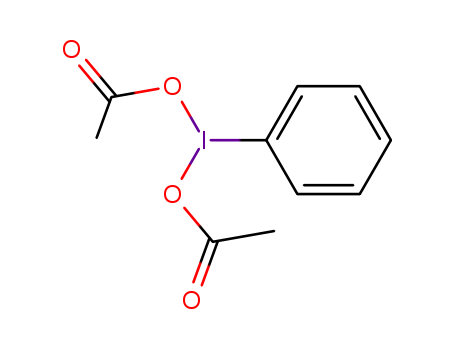

Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Purification Methods |
The purity of diacetoxyiodobenzene can be checked by treatment with H2SO4 then KI and the liberated I2 is estimated with standard thiosulfate. It has been recrystallised from 5M acetic acid and dried overnight in a vacuum desiccator over CaCl2. The surface of the crystals may become slightly yellow but this does not affect its usefulness. [Sharefkin & Saltzman Org Synth Coll Vol V 600 1973, Beilstein 5 IV 693.] |
|
General Description |
(Diacetoxyiodo)benzene, also known as iodobenzene diacetate (IBD or PIA), is a versatile hypervalent iodine(III) reagent widely used in organic synthesis for oxidative transformations. It serves as an efficient oxidant and mediator in various reactions, including cycloisomerization-amination sequences, oxidative dearomatization, halogenation of indoles, and oxidative alkyl shifts. It is particularly valued for its ability to facilitate metal-free reactions under mild conditions, enabling the synthesis of complex heterocycles, functionalized scaffolds, and bioactive molecules. Additionally, it acts as a key reagent in oxidative cyclizations, intramolecular diaminations, and the preparation of α-acetoxy ketones. Its applications span diverse synthetic strategies, including the construction of natural product skeletons and antimicrobial agents, highlighting its broad utility in modern organic chemistry. |
InChI:InChI=1/C6H5I.2C2H4O2/c7-6-4-2-1-3-5-6;2*1-2(3)4/h1-5H;2*1H3,(H,3,4)
A one-pot method for the synthesis of ar...
An anodic oxidation enabled synthesis of...
A novel approach to the preparation of i...
The invention provides a method for prod...
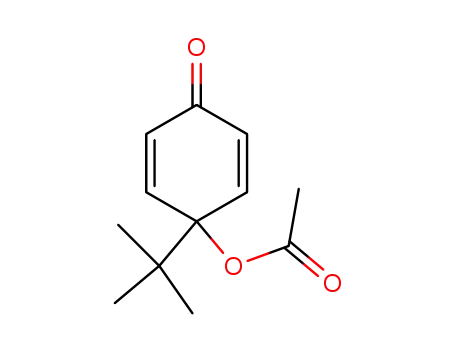
4-acetoxy-4-tert-butyl-2,5-cyclohexadienone

![[bis(acetoxy)iodo]benzene](/upload/2025/4/ba531a56-fedc-4b29-9f05-4fdd222745a9.png)
[bis(acetoxy)iodo]benzene

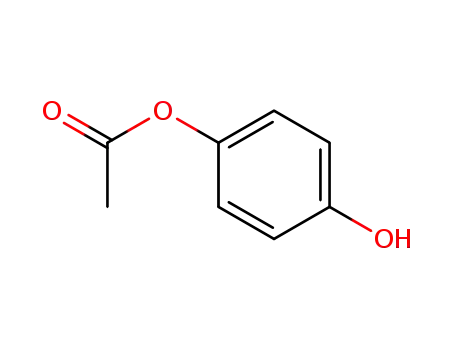
hydroquinone monoacetate

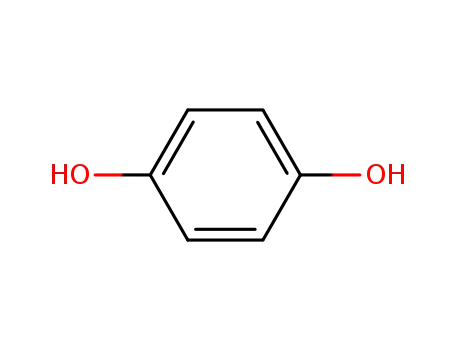
hydroquinone

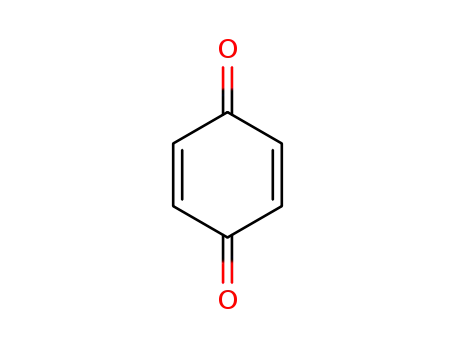
p-benzoquinone
| Conditions | Yield |
|---|---|
|
With
perchloric acid;
In
water; acetonitrile;
at 30 ℃;
pH=1.0;
Further Variations:;
pH-values;
Reagents;
Temperatures;
Kinetics;
|
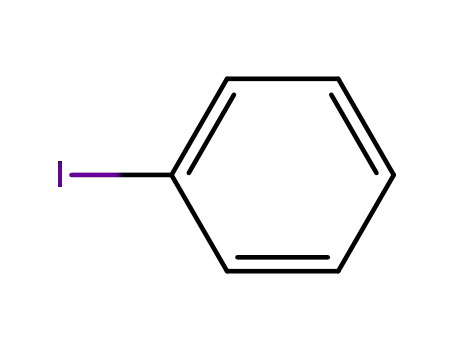
iodobenzene

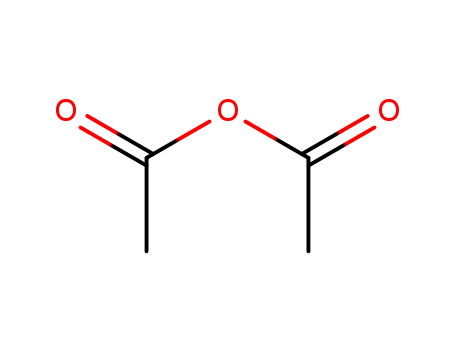
acetic anhydride

![[bis(acetoxy)iodo]benzene](/upload/2025/4/ba531a56-fedc-4b29-9f05-4fdd222745a9.png)
[bis(acetoxy)iodo]benzene
| Conditions | Yield |
|---|---|
|
With
sodium perborate hexahydrate; acetic acid;
at 55 ℃;
Inert atmosphere;
|
91% |
|
acetic anhydride;
With
dihydrogen peroxide;
In
water;
at 40 ℃;
for 4h;
iodobenzene;
at 20 - 40 ℃;
|
83% |
|
With
sodium percarbonate; acetic acid;
In
dichloromethane;
at 25 - 40 ℃;
for 6.5h;
|
79% |
|
With
sodium periodate; sodium acetate;
In
acetic acid;
for 2h;
Heating;
|
73% |
|
acetic anhydride;
With
dihydrogen peroxide;
In
water;
at 40 ℃;
for 4h;
iodobenzene;
In
water;
at 40 ℃;
|
71% |
|
With
sodium hypochlorite pentahydrate;
In
acetonitrile;
at 20 ℃;
for 0.166667h;
|
70% |
|
With
dihydrogen peroxide;
at 40 ℃;
for 4h;
|
62% |
|
With
urea-hydrogen peroxide; sodium acetate; acetic acid;
at 40 ℃;
for 3.5h;
|
44% |
|
With
dihydrogen peroxide;
|
|
|
With
dihydrogen peroxide;
|
|
|
With
dihydrogen peroxide;
at 40 ℃;
for 4h;
|
|
|
acetic anhydride;
With
dihydrogen peroxide;
at 40 ℃;
for 4h;
iodobenzene;
Further stages.;
|
23 g |
|
With
sodium periodate; sodium acetate; acetic acid;
for 2h;
Reflux;
|
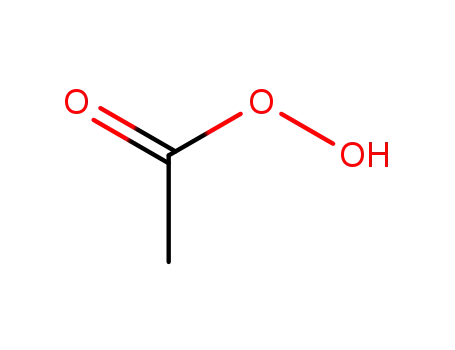
peracetic acid
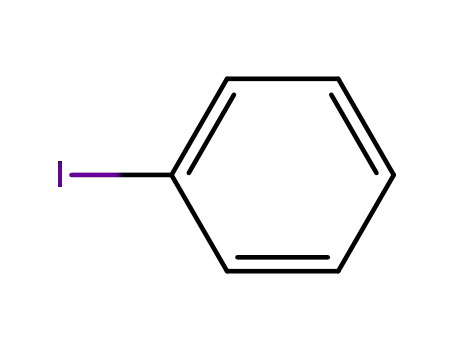
iodobenzene
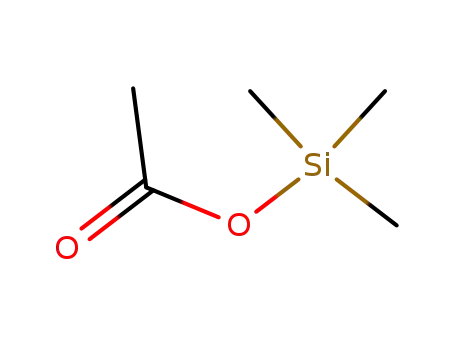
trimethylsilyl acetate
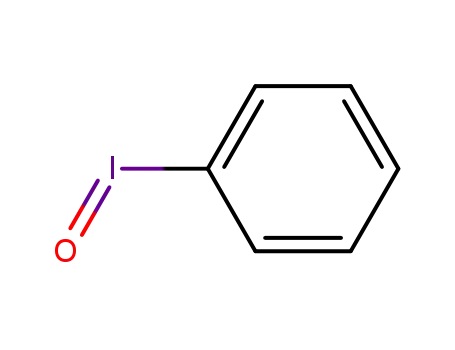
iodosylbenzene
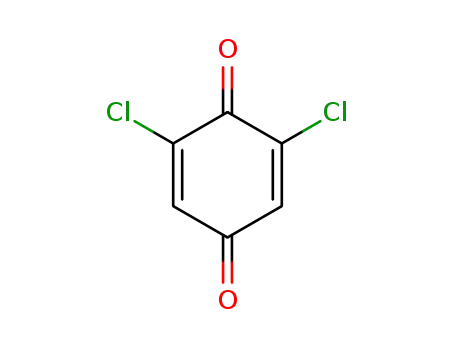
2,6-dichloro-1,4-benzoquinone
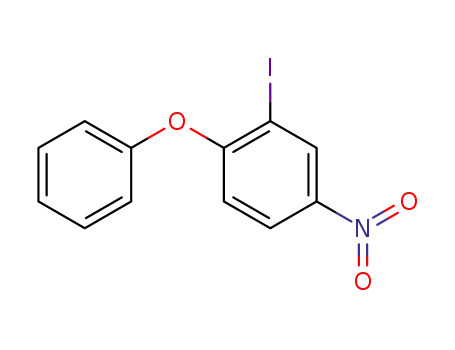
2-iodo-4-nitro-1-phenoxybenzene
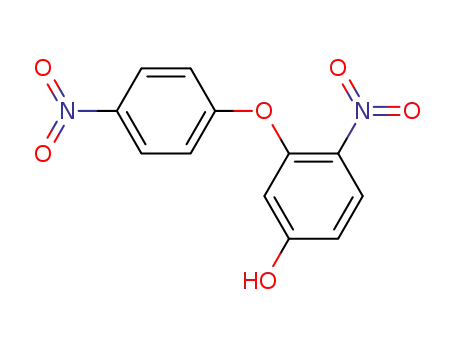
4-Nitro-3-(4-nitro-phenoxy)-phenol
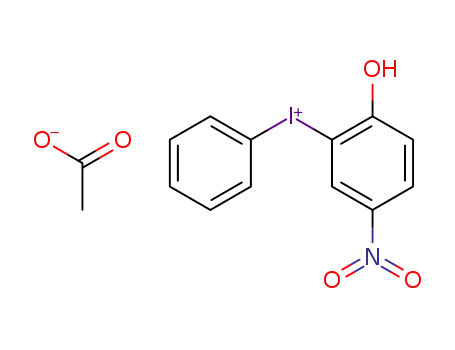
(2-hydroxy-5-nitro-phenyl)-phenyl-iodonium ; acetate
