<ol id="33bym"></ol> 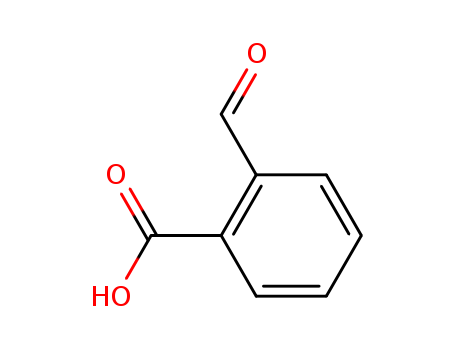

Contact Us: +86-15508631887(WhatsApp/WeChat)
Email:sales@finerchem.com
|
Synthesis Reference(s) |
Tetrahedron Letters, 14, p. 3535, 1973 DOI: 10.1016/S0040-4039(01)86963-XThe Journal of Organic Chemistry, 32, p. 3200, 1967 DOI: 10.1021/jo01285a061 |
|
General Description |
2-Carboxybenzaldehyde (also known as 2-formylbenzoic acid) serves as a key reactant in the Ugi four-component condensation (Ugi-4CC) to form stable primary adducts, which can be further transformed into isocoumarins. Its reactivity with phenacylamine dimethyl acetal and isocyanides under controlled conditions allows for the isolation and characterization of intermediates, demonstrating its utility in synthetic routes toward biologically relevant heterocycles. The compound's stability in solid state and tendency to rearrange in solution highlight its role in facilitating efficient and tunable chemical transformations. |
|
Definition |
ChEBI: An aldehydic acid which consists of benzoic acid substituted by a formyl group at position 2. |
InChI:InChI=1/C8H6O3/c9-5-6-3-1-2-4-7(6)8(10)11/h1-5H,(H,10,11)/p-1
-
-
-
Presented here is an investigation of th...
-
-
-
-
-
1,2-, 1,3-, and 1,4-Butanediols and phth...
-
An efficient redox-amination-aromatizati...
Rate constants were measured for the oxi...
The invention provides an industrial syn...
The invention discloses a synthesis meth...
A Zn-mediated propargylation/lactamizati...
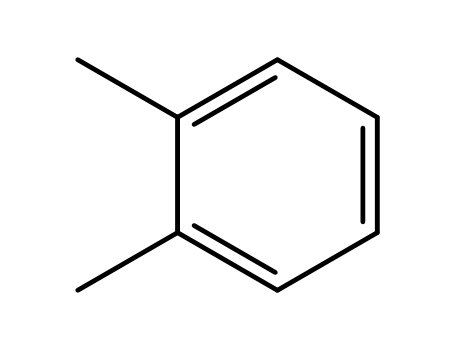
o-xylene

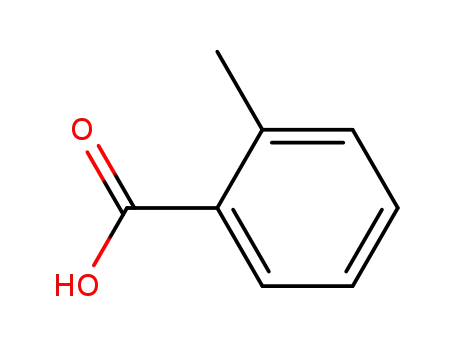
ortho-methylbenzoic acid

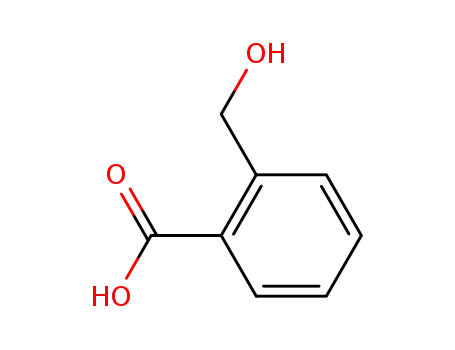
2-(hydroxymethyl)benzoic acid

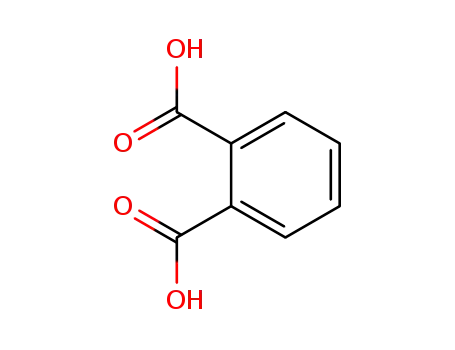
benzene-1,2-dicarboxylic acid

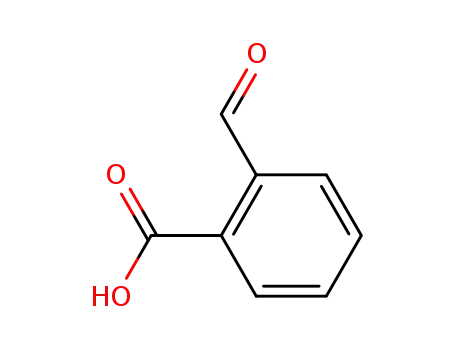
o-carboxybenzaldehyde
| Conditions | Yield |
|---|---|
|
o-xylene;
With
N-hydroxyphthalimide; air;
cobalt(II) acetate; manganese(II) acetate;
In
acetic acid;
at 150 ℃;
for 3h;
under 22801.5 Torr;
With
sodium hydroxide;
at 90 ℃;
Further stages.;
|
73% 6% 8% 3% |

o-xylene


ortho-methylbenzoic acid

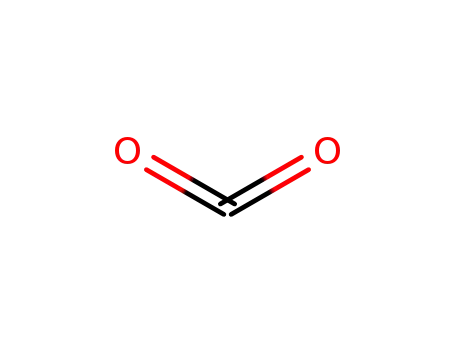
carbon dioxide


2-(hydroxymethyl)benzoic acid


benzene-1,2-dicarboxylic acid

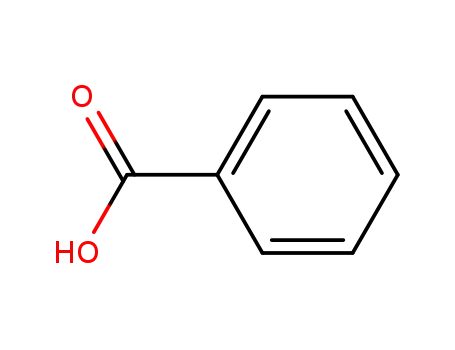
benzoic acid


o-carboxybenzaldehyde
| Conditions | Yield |
|---|---|
|
With
water; hydrogen bromide; dihydrogen peroxide;
at 380 ℃;
under 172517 Torr;
|
58.6 mol % 15.2 mol % |
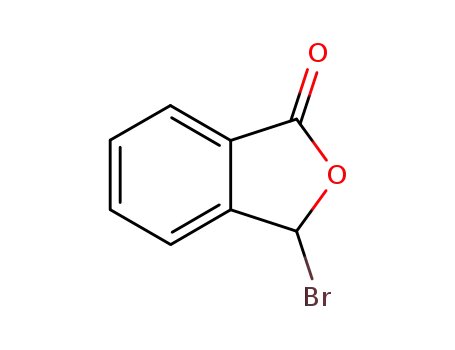
3-Bromophthalide
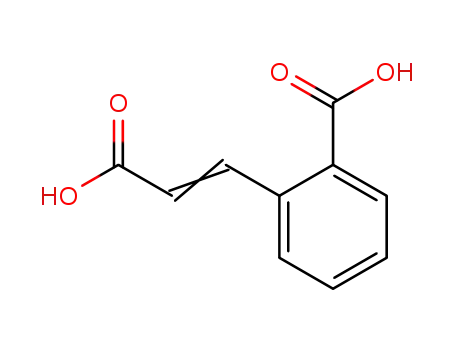
2-carboxycinnamic acid
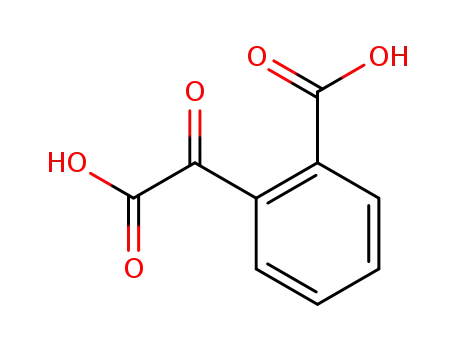
phthalonic acid
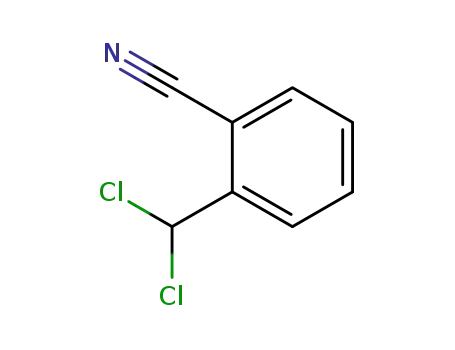
2-dichloromethyl-benzonitrile
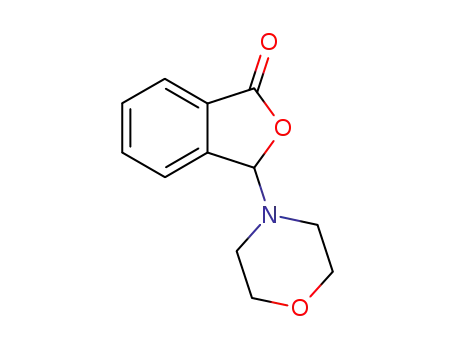
3-morpholin-4-yl-3H-isobenzofuran-1-one
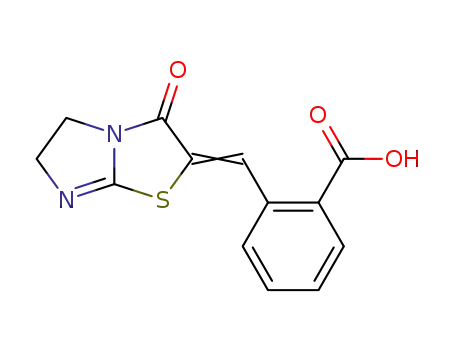
2-(3-oxo-5,6-dihydro-imidazo[2,1-b]thiazol-2-ylidenemethyl)-benzoic acid
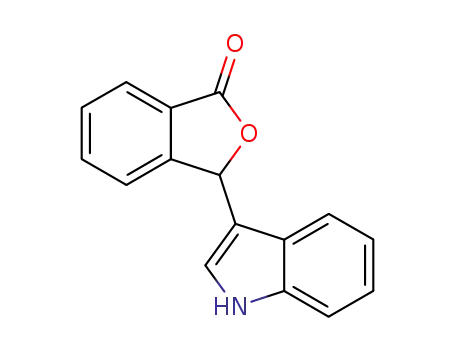
3-(1H-indol-3-yl)isobenzofuran-1(3H)-one
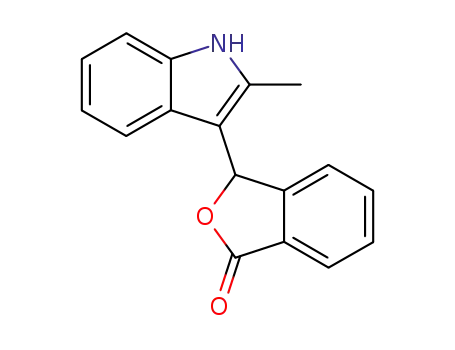
2-Methyl-3-phthalidyl-indol
